One-pot HTST synthesis of responsive fluorescent ZnO@apo-enzyme composite microgels for intracellular glucometry
- PMID: 35519737
- PMCID: PMC9055424
- DOI: 10.1039/d0ra04339g
One-pot HTST synthesis of responsive fluorescent ZnO@apo-enzyme composite microgels for intracellular glucometry
Abstract
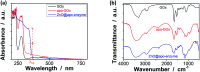
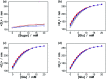
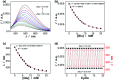
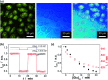
Responsive fluorescent microgels, that can selectively, reversibly, and rapidly convert the fluctuation in intracellular glucose level into fluorescence signal, have the potential use for intracellular glucometry to promote the understanding of physiology. Herein, we report one-pot synthesis of such a responsive fluorescent composite microgels, which is made of a representative apo-enzyme, apo-glucose oxidase (apo-GOx), interpenetrated in a composite gel network that is comprised of ZnO quantum dots covalently bonded onto crosslinked poly(ethylene glycol) dimethacrylate. The key of this one-pot synthesis is applying a high-temperature short-time heating (HTST) method, so that the naturally dynamic profile of apo-GOx can be maintained and harnessed on the composite microgels to allow the highly selective response to glucose over a glucose concentration range of 0-20 mM. While the composite microgels can undergo volume phase transitions and convert both an increase and a decrease in glucose concentration into fluorescence signal shortly (<1 s), the changes in average hydrodynamic diameter and fluorescence of the composite microgels can be fully reversible even after twenty cycles of adding/removing glucose, indicating a reversible and rapid time response to the glucose concentration variations. With the composite microgels as biosensors, the fluorescence of the composite microgels embedded in the model cancer cells B16F10 can be modulated in response to intracellular glucose level variations, which are derived from a change in glucose concentration in the culture medium by an external supply, or that can be triggered by biochemical reactions (with the β-galactosidase catalysed hydrolysis of lactose as a model reaction for achieving increased glucose levels, and the GOx catalysed oxidation of glucose for achieving decreased glucose levels).
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures








Similar articles
-
A fluorescent double-network-structured hybrid nanogel as embeddable nanoglucometer for intracellular glucometry.Biomater Sci. 2013 Apr 5;1(4):421-433. doi: 10.1039/c2bm00162d. Epub 2013 Jan 10. Biomater Sci. 2013. PMID: 32481907
-
Immobilization of Carbon Dots in Molecularly Imprinted Microgels for Optical Sensing of Glucose at Physiological pH.ACS Appl Mater Interfaces. 2015 Jul 29;7(29):15735-45. doi: 10.1021/acsami.5b04744. Epub 2015 Jul 17. ACS Appl Mater Interfaces. 2015. PMID: 26148139
-
Composite hydrogels with temperature sensitive heterogeneities: influence of gel matrix on the volume phase transition of embedded poly-(N-isopropylacrylamide) microgels.Phys Chem Chem Phys. 2011 Feb 28;13(8):3039-47. doi: 10.1039/c0cp01135e. Epub 2010 Sep 30. Phys Chem Chem Phys. 2011. PMID: 20882241
-
Fluorescent Polymer-MoS2-Embedded Microgels for Photothermal Heating and Colorimetric Monitoring.ACS Appl Mater Interfaces. 2020 Aug 5;12(31):35415-35423. doi: 10.1021/acsami.0c08125. Epub 2020 Jul 23. ACS Appl Mater Interfaces. 2020. PMID: 32662977
-
Thermal sensitive microgels with stable and reversible photoluminescence based on covalently bonded quantum dots.Langmuir. 2010 Apr 6;26(7):5022-7. doi: 10.1021/la903667r. Langmuir. 2010. PMID: 20201483
Cited by
-
Design and Synthesis of Novel Di-Boronic Acid-Based Chemical Glucose Sensors.ACS Omega. 2025 Mar 11;10(11):10812-10825. doi: 10.1021/acsomega.4c06237. eCollection 2025 Mar 25. ACS Omega. 2025. PMID: 40160773 Free PMC article.
References
LinkOut - more resources
Full Text Sources

