X-ray absorption spectroscopy of organic sulfoxides
- PMID: 35519739
- PMCID: PMC9055334
- DOI: 10.1039/d0ra04653a
X-ray absorption spectroscopy of organic sulfoxides
Abstract
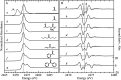
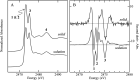
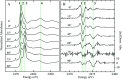
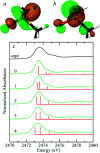
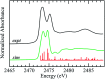
Organic sulfoxides, a group of compounds containing the sulfinyl S[double bond, length as m-dash]O group, are widespread in nature, important in health and disease, and used in a variety of applications in the pharmaceutical industry. We have examined the sulfur K-edge X-ray absorption near-edge spectra of a range of different sulfoxides and find that their spectra are remarkably similar. Spectra show an intense absorption peak that is comprised of two transitions; a S 1s → (S-O)σ* and a S 1s → [(S-O)π* + (S-C)σ*] transition. In most cases these are sufficiently close in energy that they are not properly resolved; however for dimethylsulfoxide the separation between these transitions increases in aqueous solution due to hydrogen bonding to the sulfinyl oxygen. We also examined tetrahydrothiophene sulfoxide using both the sulfur and oxygen K-edge. This compound has a mild degree of ring strain at the sulfur atom, which changes the energies of the two transitions so that the S 1s → [(S-O)π* + (S-C)σ*] is below the S 1s → (S-O)σ*. A comparison of the oxygen K-edge X-ray absorption near-edge spectra of tetrahydrothiophene sulfoxide with that of an unhindered sulfoxide shows little change, indicating that the electronic environment of oxygen is very similar.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
Theoretical and experimental sulfur K-edge X-ray absorption spectroscopic study of cysteine, cystine, homocysteine, penicillamine, methionine and methionine sulfoxide.Dalton Trans. 2009 May 14;(18):3542-58. doi: 10.1039/b819257j. Epub 2009 Mar 10. Dalton Trans. 2009. PMID: 19381417
-
X-ray Absorption Spectroscopy of Aliphatic Organic Sulfides.J Phys Chem A. 2017 Aug 24;121(33):6256-6261. doi: 10.1021/acs.jpca.7b04395. Epub 2017 Aug 10. J Phys Chem A. 2017. PMID: 28753014
-
Chemical Sensitivity of the Sulfur K-Edge X-ray Absorption Spectra of Organic Disulfides.J Phys Chem A. 2016 Sep 22;120(37):7279-86. doi: 10.1021/acs.jpca.6b06790. Epub 2016 Sep 8. J Phys Chem A. 2016. PMID: 27571342
-
Sulfur X-ray absorption and vibrational spectroscopic study of sulfur dioxide, sulfite, and sulfonate solutions and of the substituted sulfonate ions X3CSO3- (X = H, Cl, F).Inorg Chem. 2007 Oct 1;46(20):8332-48. doi: 10.1021/ic062440i. Epub 2007 Sep 5. Inorg Chem. 2007. PMID: 17784748
-
Ambidentate coordination in hydrogen bonded dimethyl sulfoxide, (CH3)2SO...H3O+, and in dichlorobis(dimethyl sulfoxide) palladium(II) and platinum(II) solid solvates, by vibrational and sulfur K-edge X-ray absorption spectroscopy.Dalton Trans. 2009 Feb 28;(8):1328-38. doi: 10.1039/b814252a. Dalton Trans. 2009. PMID: 19462654
Cited by
-
Determination of Thiol Protonation States by Sulfur X-ray Spectroscopy in Biological Systems.J Phys Chem Lett. 2025 Mar 6;16(9):2401-2408. doi: 10.1021/acs.jpclett.4c03247. Epub 2025 Feb 26. J Phys Chem Lett. 2025. PMID: 40012333
-
Local and Symmetry-Resolved Electronic Structure of Liquid Dimethyl Sulfoxide from Resonant Inelastic Soft X-ray Scattering.J Phys Chem Lett. 2024 Oct 24;15(42):10576-10582. doi: 10.1021/acs.jpclett.4c02329. Epub 2024 Oct 14. J Phys Chem Lett. 2024. PMID: 39401783
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

