Theoretical investigation of the reaction mechanisms and kinetics of CFCl2CH2O2 and ClO in the atmosphere
- PMID: 35519771
- PMCID: PMC9055410
- DOI: 10.1039/d0ra04707d
Theoretical investigation of the reaction mechanisms and kinetics of CFCl2CH2O2 and ClO in the atmosphere
Abstract
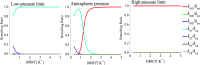
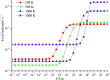
The reaction between CFCl2CH2O2 radicals and ClO was studied using the B3LYP and CCSD(T) methods associated with the 6-311++G(d,p) and cc-pVTZ basis sets, and subsequently RRKM-TST theory was used to predict the thermal rate constants and product distributions. On the singlet PES, the dominant reaction is the addition of the ClO oxygen atom to the terminal-O of CFCl2CH2O2 to generate adduct IM1 (CFCl2CH2OOOCl), and then dissociation to final products P1 (CFCl2CHO + HO2 + Cl) occurs. RRKM theory is employed to calculate the overall and individual rate constants over a wide range of temperatures and pressures. It is predicted that the collision-stabilized IM1 (CFCl2CH2OOOCl) dominates the reaction at 200-500 K (accounting for about 60-100%) and the dominant products are P1 (CFCl2CHO + HO2 + Cl). The yields of the other products are very low and insignificant for the title reaction. The total rate constants exhibit typical "falloff" behavior. The pathways on the triplet PES are less competitive than that on the singlet PES. The calculated overall rate constants are in good agreement with the experimental data. The atmospheric lifetime of CFCl2CH2O2 in ClO is around 2.04 h. TD-DFT calculations imply that IM1 (CFCl2CH2OOOCl), IM2 (CFCl2CH2OOClO) and IM3 (CFCl2CH2OClO2) will photolyze under sunlight.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







Similar articles
-
Computational study on mechanisms and kinetics of the atmospheric CFCl2CH2O2 with Cl reaction.J Mol Graph Model. 2020 Sep;99:107618. doi: 10.1016/j.jmgm.2020.107618. Epub 2020 Apr 17. J Mol Graph Model. 2020. PMID: 32339900
-
Theoretical investigations on mechanisms and kinetics of the CH3CFClO2· with ClO· reaction in the atmosphere.Sci Rep. 2020 Jul 6;10(1):11078. doi: 10.1038/s41598-020-68049-4. Sci Rep. 2020. PMID: 32632199 Free PMC article.
-
Computational study on the mechanisms and kinetics of the CH2BrO2 + ClO reaction in the atmosphere.RSC Adv. 2020 Jun 25;10(41):24308-24318. doi: 10.1039/c9ra10511e. eCollection 2020 Jun 24. RSC Adv. 2020. PMID: 35516180 Free PMC article.
-
Theoretical investigations on mechanisms and pathways of CH2ClO2/CHCl2O2 with ClO reactions in the atmosphere.Environ Sci Pollut Res Int. 2020 Jun;27(16):20457-20468. doi: 10.1007/s11356-020-08315-0. Epub 2020 Apr 3. Environ Sci Pollut Res Int. 2020. PMID: 32242320
-
Atmospheric chemistry of CFCl2O2: a theoretical study on mechanisms and kinetics of the CFCl2O2 + ClO reaction.J Mol Model. 2020 Jun 15;26(7):177. doi: 10.1007/s00894-020-04432-2. J Mol Model. 2020. PMID: 32542446
References
-
- Tuazon E. C. and Atkinson R., Potential Replacements for the Chlorofluorocarbons and Halons, American Chemical Society, Washington, DC, 1995
-
- Sehested J. Int. J. Chem. Kinet. 1994;26:1023–1039. doi: 10.1002/kin.550261007. - DOI
-
- Atkinson R. Baulch D. L. Cox R. A. Hampson Jr R. F. Kerr J. A. Rossi M. J. Troe J. J. Phys. Chem. Ref. Data. 1997;26:521–1011. doi: 10.1063/1.556011. - DOI
-
- Lightfoot P. D. Cox R. A. Crowley J. N. Destriau M. Hayman G. D. Jenkin M. E. Moortgat G. K. Zabel F. Atmos. Environ., Part A. 1992;26:1805–1961. doi: 10.1016/0960-1686(92)90423-I. - DOI
-
- Wallington T. J. Nielsen O. J. Int. J. Chem. Kinet. 1991;23:785–798. doi: 10.1002/kin.550230905. - DOI
LinkOut - more resources
Full Text Sources

