Stable Isotope Fractionation of Metals and Metalloids in Plants: A Review
- PMID: 35519812
- PMCID: PMC9063737
- DOI: 10.3389/fpls.2022.840941
Stable Isotope Fractionation of Metals and Metalloids in Plants: A Review
Abstract
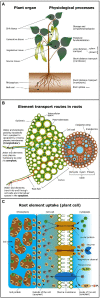
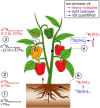
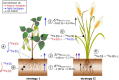
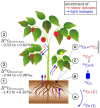
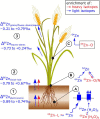
This work critically reviews stable isotope fractionation of essential (B, Mg, K, Ca, Fe, Ni, Cu, Zn, Mo), beneficial (Si), and non-essential (Cd, Tl) metals and metalloids in plants. The review (i) provides basic principles and methodologies for non-traditional isotope analyses, (ii) compiles isotope fractionation for uptake and translocation for each element and connects them to physiological processes, and (iii) interlinks knowledge from different elements to identify common and contrasting drivers of isotope fractionation. Different biological and physico-chemical processes drive isotope fractionation in plants. During uptake, Ca and Mg fractionate through root apoplast adsorption, Si through diffusion during membrane passage, Fe and Cu through reduction prior to membrane transport in strategy I plants, and Zn, Cu, and Cd through membrane transport. During translocation and utilization, isotopes fractionate through precipitation into insoluble forms, such as phytoliths (Si) or oxalate (Ca), structural binding to cell walls (Ca), and membrane transport and binding to soluble organic ligands (Zn, Cd). These processes can lead to similar (Cu, Fe) and opposing (Ca vs. Mg, Zn vs. Cd) isotope fractionation patterns of chemically similar elements in plants. Isotope fractionation in plants is influenced by biotic factors, such as phenological stages and plant genetics, as well as abiotic factors. Different nutrient supply induced shifts in isotope fractionation patterns for Mg, Cu, and Zn, suggesting that isotope process tracing can be used as a tool to detect and quantify different uptake pathways in response to abiotic stresses. However, the interpretation of isotope fractionation in plants is challenging because many isotope fractionation factors associated with specific processes are unknown and experiments are often exploratory. To overcome these limitations, fundamental geochemical research should expand the database of isotope fractionation factors and disentangle kinetic and equilibrium fractionation. In addition, plant growth studies should further shift toward hypothesis-driven experiments, for example, by integrating contrasting nutrient supplies, using established model plants, genetic approaches, and by combining isotope analyses with complementary speciation techniques. To fully exploit the potential of isotope process tracing in plants, the interdisciplinary expertise of plant and isotope geochemical scientists is required.
Keywords: fractionation; metalloids; metals; multiple-collector-ICP-MS; plant uptake; process tracing; stable isotopes; translocation.
Copyright © 2022 Wiggenhauser, Moore, Wang, Bienert, Laursen and Blotevogel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Stable isotopes of Cu and Zn in higher plants: evidence for Cu reduction at the root surface and two conceptual models for isotopic fractionation processes.Environ Sci Technol. 2012 Mar 6;46(5):2652-60. doi: 10.1021/es202587m. Epub 2012 Feb 10. Environ Sci Technol. 2012. PMID: 22296233
-
Metal stable isotopes fractionation during adsorption.Ecotoxicol Environ Saf. 2024 Sep 15;283:116770. doi: 10.1016/j.ecoenv.2024.116770. Epub 2024 Jul 26. Ecotoxicol Environ Saf. 2024. PMID: 39067077 Review.
-
Nickel and zinc isotope fractionation in hyperaccumulating and nonaccumulating plants.Environ Sci Technol. 2014 Oct 21;48(20):11926-33. doi: 10.1021/es5020955. Epub 2014 Sep 30. Environ Sci Technol. 2014. PMID: 25222693
-
Metal stable isotope signatures as tracers in environmental geochemistry.Environ Sci Technol. 2015 Mar 3;49(5):2606-24. doi: 10.1021/es504683e. Epub 2015 Feb 17. Environ Sci Technol. 2015. PMID: 25640608 Review.
-
Zinc uptake and replenishment mechanisms during repeated phytoextraction using Sedum plumbizincicola revealed by stable isotope fractionation.Sci Total Environ. 2022 Feb 1;806(Pt 3):151306. doi: 10.1016/j.scitotenv.2021.151306. Epub 2021 Oct 29. Sci Total Environ. 2022. PMID: 34743872
Cited by
-
From soil to cacao bean: Unravelling the pathways of cadmium translocation in a high Cd accumulating cultivar of Theobroma cacao L.Front Plant Sci. 2022 Dec 2;13:1055912. doi: 10.3389/fpls.2022.1055912. eCollection 2022. Front Plant Sci. 2022. PMID: 36531371 Free PMC article.
-
Coupling metal stable isotope compositions and X-ray absorption spectroscopy to study metal pathways in soil-plant systems: a mini review.Metallomics. 2023 Apr 3;15(4):mfad016. doi: 10.1093/mtomcs/mfad016. Metallomics. 2023. PMID: 36893801 Free PMC article. Review.
-
Amino Acid Complexation Fractionates Nickel Isotopes: Implications for Tracing Nickel Cycling in the Environment.Environ Sci Technol Lett. 2025 Feb 27;12(3):283-288. doi: 10.1021/acs.estlett.4c01060. eCollection 2025 Mar 11. Environ Sci Technol Lett. 2025. PMID: 40093648 Free PMC article.
-
An investigation of zinc isotope fractionation in cacao (Theobroma cacao L.) and comparison of zinc and cadmium isotope compositions in hydroponic plant systems under high cadmium stress.Sci Rep. 2023 Mar 22;13(1):4682. doi: 10.1038/s41598-023-30899-z. Sci Rep. 2023. PMID: 36949227 Free PMC article.
-
In Vitro Fossilization for High Spatial Resolution Quantification of Elements in Plant-Tissue Using LA-ICP-TOFMS.Anal Chem. 2024 Mar 26;96(12):4952-4959. doi: 10.1021/acs.analchem.3c05849. Epub 2024 Mar 14. Anal Chem. 2024. PMID: 38482755 Free PMC article.
References
-
- Arnold T., Markovic T., Kirk G. J. D., Schönbächler M., Rehkämper M., Zhao F. J., et al. . (2015). Iron and zinc isotope fractionation during uptake and translocation in rice (Oryza sativa) grown in oxic and anoxic soils. C. R. Geosci. 347, 397–404. doi: 10.1016/j.crte.2015.05.005 - DOI
-
- Aucour A. M., Bedell J. P., Queyron M., Magnin V., Testemale D., Sarret G. (2015). Dynamics of Zn in an urban wetland soil-plant system: coupling isotopic and EXAFS approaches. Geochim. Cosmochim. Acta 160, 55–69. doi: 10.1016/j.gca.2015.03.040 - DOI
Publication types
LinkOut - more resources
Full Text Sources

