Copper-catalyzed intramolecular cross dehydrogenative coupling approach to coumestans from 2'-hydroxyl-3-arylcoumarins
- PMID: 35519854
- PMCID: PMC9064580
- DOI: 10.1039/c9ra01909j
Copper-catalyzed intramolecular cross dehydrogenative coupling approach to coumestans from 2'-hydroxyl-3-arylcoumarins
Abstract
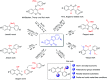
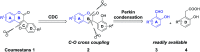
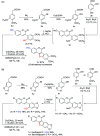
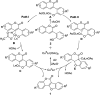
A copper-catalyzed intramolecular cross dehydrogenative C-O coupling reaction of 2'-hydroxyl-3-arylcoumarins was developed. This protocol provided a facile and efficient strategy for the construction of natural coumestans and derivatives in moderate to high yields. This transformation exhibited good functional group compatibility and was amenable to substrates with free phenolic hydroxyl groups.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Synthesis of Phenolic Coumestans via a Sequential Dehydrogenation/Oxa-Michael Addition Reaction of 2',4'-Dihydroxyl-3-arylcoumarins.J Org Chem. 2022 May 6;87(9):5785-5794. doi: 10.1021/acs.joc.2c00120. Epub 2022 Apr 14. J Org Chem. 2022. PMID: 35420815
-
Copper-catalyzed diastereoselective aerobic intramolecular dehydrogenative coupling of hydrazones via sp3 C-H functionalization.Chem Sci. 2015 Oct 1;6(10):5882-5890. doi: 10.1039/c5sc01736j. Epub 2015 Jul 14. Chem Sci. 2015. PMID: 29861913 Free PMC article.
-
Cobalt(III)-Catalyzed Intramolecular Cross-Dehydrogenative C-H/X-H Coupling: Efficient Synthesis of Indoles and Benzofurans.Chemistry. 2016 Nov 2;22(45):16042-16046. doi: 10.1002/chem.201604111. Epub 2016 Sep 30. Chemistry. 2016. PMID: 27608137
-
Progress in copper-catalysed/mediated intramolecular dehydrogenative coupling.Org Biomol Chem. 2023 Jan 4;21(2):237-251. doi: 10.1039/d2ob01796b. Org Biomol Chem. 2023. PMID: 36448561 Review.
-
Metal-Free Cross-Dehydrogenative Coupling (CDC): Molecular Iodine as a Versatile Catalyst/Reagent for CDC Reactions.Chem Asian J. 2019 Jan 4;14(1):6-30. doi: 10.1002/asia.201801237. Epub 2018 Nov 2. Chem Asian J. 2019. PMID: 30259704 Review.
Cited by
-
Progress and prospects in copper-catalyzed C-H functionalization.RSC Adv. 2020 Sep 17;10(57):34429-34458. doi: 10.1039/d0ra06518h. eCollection 2020 Sep 16. RSC Adv. 2020. PMID: 35514395 Free PMC article. Review.
-
Total Syntheses and Anti-Inflammatory Studies of Three Natural Coumarins: Glycycoumarin, Glycyrin, and 3-O-Methylglycyrol.Molecules. 2024 Aug 21;29(16):3942. doi: 10.3390/molecules29163942. Molecules. 2024. PMID: 39203020 Free PMC article.
-
Metal-free C-3 selective C(sp2)-C(sp3) heteroarylation of anilines with imidazo[1,2-a]pyridine derivatives via cross-dehydrogenative coupling.RSC Adv. 2023 Jul 19;13(31):21685-21689. doi: 10.1039/d3ra03852a. eCollection 2023 Jul 12. RSC Adv. 2023. PMID: 37476046 Free PMC article.
References
-
- Bickoff E. M. Lyman R. L. Livingston A. L. Booth A. N. J. Am. Chem. Soc. 1958;80:3969–3971. doi: 10.1021/ja01548a043. - DOI
- Xi G.-L. Liu Z.-Q. J. Agric. Food Chem. 2014;62:5636–5642. doi: 10.1021/jf500013v. - DOI - PubMed
- Tuskaev V. A. Pharm. Chem. J. 2013;47:1–11. doi: 10.1007/s11094-013-0886-5. - DOI
- Zhang W. Lun S. Wang S. Jiang X. Yang F. Tang J. Manson A. L. Earl A. M. Gunosewoyo H. Bishai W. R. Yu L.-F. J. Med. Chem. 2018;61:791–803. doi: 10.1021/acs.jmedchem.7b01319. - DOI - PubMed
-
- Xia Y. Chen J. Cao Y. Xu C. Li R. Pan Y. Chen X. Eur. J. Pharmacol. 2013;714:105–111. doi: 10.1016/j.ejphar.2013.06.012. - DOI - PubMed
- Lim S. Jang H.-J. Park E. H. Kim J. K. Kim J.-M. Kim E.-K. Yea K. Kim Y.-H. Lee-Kwon W. Ryu S. H. Suh P.-G. J. Cell. Biochem. 2012;113:3436–3445. doi: 10.1002/jcb.24220. - DOI - PubMed
- Li L. Deng X. Zhang L. Shu P. Qin M. Fitoterapia. 2011;82:615–619. doi: 10.1016/j.fitote.2011.01.019. - DOI - PubMed
- Chen Y. Wei X. Xie H. Deng H. J. Nat. Prod. 2008;71:929–932. doi: 10.1021/np800016e. - DOI - PubMed
- Xu M.-Y. Kim Y.-S. Food Chem. Toxicol. 2014;74:311–319. doi: 10.1016/j.fct.2014.10.023. - DOI - PubMed
- Nehybova T. Smarda J. Daniel L. Brezovsky J. Benes P. J. Steroid Biochem. Mol. Biol. 2015;152:76–83. doi: 10.1016/j.jsbmb.2015.04.019. - DOI - PubMed
- DaSilva A. J. M. Melo P. A. Silva N. M. V. Brito F. V. Buarque C. D. de Souza D. V. Rodrigues V. P. Pocas E. S. C. Noel F. Albuquerque E. X. Costa P. R. R. Bioorg. Med. Chem. Lett. 2001;11:283–286. doi: 10.1016/S0960-894X(00)00621-1. - DOI - PubMed
-
- Mackey K. Pardo L. M. Prendergast A. M. Nolan M.-T. Bateman L. M. McGlacken G. P. Org. Lett. 2016;18:2540–2543. doi: 10.1021/acs.orglett.6b00751. - DOI - PubMed
- Neog K. Borah A. Gogoi P. J. Org. Chem. 2016;81:11971–11977. doi: 10.1021/acs.joc.6b01966. - DOI - PubMed
- Kapdi A. R. Karbelkar A. Naik M. Pednekar S. Fischer C. Schulzkec C. Tromp M. RSC Adv. 2013;3:20905–20912. doi: 10.1039/C3RA43821J. - DOI
- Tang L. Pang Y. Yan Q. Shi L. Huang J. Du Y. Zhao K. J. Org. Chem. 2011;76:2744–2752. doi: 10.1021/jo2000644. - DOI - PubMed
- Kshirsagar U. A. Parnes R. Goldshtein H. Ofir R. Zarivach R. Pappo D. Chem.–Eur. J. 2013;19:13575–13583. doi: 10.1002/chem.201300389. - DOI - PubMed
- Liu J. Liu Y. Du W. Dong Y. Liu J. Wang M. J. Org. Chem. 2013;78:7293–7297. doi: 10.1021/jo400984h. - DOI - PubMed
- Zhang J. Qiu J. Xiao C. Yu L. Yang F. Tang J. Eur. J. Org. Chem. 2016:3380–3385. doi: 10.1002/ejoc.201600122. - DOI
- Yao T. Yue D. Larock R. C. J. Org. Chem. 2005;70:9985–9989. doi: 10.1021/jo0517038. - DOI - PubMed
- Nolan M. T. Pardo L. M. Prendergast A. M. McGlacken G. P. J. Org. Chem. 2015;80:10904–10913. doi: 10.1021/acs.joc.5b02027. - DOI - PubMed
- Cheng C. Chen W. Xu B. Xu M.-H. Org. Chem. Front. 2016;3:1111–1115. doi: 10.1039/C6QO00270F. - DOI
- Kurosawa K. Nogami K. Bull. Chem. Soc. Jpn. 1976;49:1955–1957. doi: 10.1246/bcsj.49.1955. - DOI
- Mali R. S. Tilve S. G. Synth. Commun. 1990;20:1781–1791. doi: 10.1080/00397919008053103. - DOI
- Naik M. Kamat V. P. Tilve S. G. Tetrahedron. 2017;73:5528–5536. doi: 10.1016/j.tet.2017.07.057. - DOI
- Chang C.-F. Yang L.-Y. Chang S.-W. Fang Y.-T. Lee Y.-J. Tetrahedron. 2008;64:3661–3666. doi: 10.1016/j.tet.2008.02.031. - DOI
- Gong D. H. Li C. Z. Yuan C. Y. Chin. J. Chem. 2001;19:522–527. doi: 10.1002/cjoc.20010190517. - DOI
- Pandit S. B. Gadre S. Y. Synth. Commun. 1988;18:157–166. doi: 10.1080/00397918808077340. - DOI
-
- Li C. C. Xie Z. X. Zhang Y. D. Chen J. H. Yang Z. J. Org. Chem. 2003;68:8500–8504. doi: 10.1021/jo030228f. - DOI - PubMed
- Yao T. Yue D. Larock R. C. J. Org. Chem. 2005;70:9985–9989. doi: 10.1021/jo0517038. - DOI - PubMed
- Hiroya K. Suzuki N. Yasuhara A. Egawa Y. Kasano A. Sakamoto T. J. Chem. Soc., Perkin Trans. 1. 2000:4339–4346. doi: 10.1039/B006623K. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

