Cross-dehydrogenative coupling reactions between arenes (C-H) and carboxylic acids (O-H): a straightforward and environmentally benign access to O-aryl esters
- PMID: 35519864
- PMCID: PMC9064605
- DOI: 10.1039/c9ra01941c
Cross-dehydrogenative coupling reactions between arenes (C-H) and carboxylic acids (O-H): a straightforward and environmentally benign access to O-aryl esters
Abstract
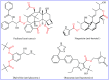
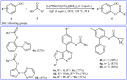
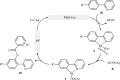
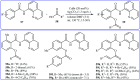
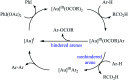
Transition-metal catalyzed cross-dehydrogenative-coupling reactions encompass highly versatile and atom economical methods for the construction of various carbon-carbon and carbon-heteroatom bonds by combining two C(X)-H (X = heteroatom) bonds. Along this line, direct acyloxylation of C-H bonds with carboxylic acids has emerged as a powerful and green approach for the synthesis of structurally diverse esters. In this focus-review we will describe recent progress in direct esterification of aromatic C-H bonds with special emphasis on the mechanistic features of the reactions. Literature has been surveyed until the end of February 2019.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



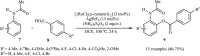
























Similar articles
-
Photocatalytic Activation of Less Reactive Bonds and Their Functionalization via Hydrogen-Evolution Cross-Couplings.Acc Chem Res. 2018 Oct 16;51(10):2512-2523. doi: 10.1021/acs.accounts.8b00267. Epub 2018 Oct 3. Acc Chem Res. 2018. PMID: 30280898
-
Cross-Dehydrogenative Coupling Reactions Between C(sp)-H and X-H (X = N, P, S, Si, Sn) Bonds: An Environmentally Benign Access to Heteroatom-Substituted Alkynes.Top Curr Chem (Cham). 2019 Jul 4;377(4):20. doi: 10.1007/s41061-019-0245-4. Top Curr Chem (Cham). 2019. PMID: 31273478 Review.
-
Mechanism-Driven Development of Group 10 Metal-Catalyzed Decarbonylative Coupling Reactions.Acc Chem Res. 2022 Dec 6;55(23):3430-3444. doi: 10.1021/acs.accounts.2c00496. Epub 2022 Nov 16. Acc Chem Res. 2022. PMID: 36382937 Free PMC article.
-
Metal-Free Cross-Dehydrogenative Coupling (CDC): Molecular Iodine as a Versatile Catalyst/Reagent for CDC Reactions.Chem Asian J. 2019 Jan 4;14(1):6-30. doi: 10.1002/asia.201801237. Epub 2018 Nov 2. Chem Asian J. 2019. PMID: 30259704 Review.
-
Upgrading Cross-Coupling Reactions for Biaryl Syntheses.Acc Chem Res. 2019 Jan 15;52(1):161-169. doi: 10.1021/acs.accounts.8b00408. Epub 2018 Oct 30. Acc Chem Res. 2019. PMID: 30376296
Cited by
-
Direct sulfonamidation of (hetero)aromatic C-H bonds with sulfonyl azides: a novel and efficient route to N-(hetero)aryl sulfonamides.RSC Adv. 2020 Oct 8;10(61):37299-37313. doi: 10.1039/d0ra04255b. eCollection 2020 Oct 7. RSC Adv. 2020. PMID: 35521237 Free PMC article. Review.
-
Recent trends in the direct oxyphosphorylation of C-C multiple bonds.RSC Adv. 2020 Dec 23;11(1):470-483. doi: 10.1039/d0ra08074h. eCollection 2020 Dec 21. RSC Adv. 2020. PMID: 35423055 Free PMC article. Review.
-
Catalytic system having an organotellurium ligand on graphene oxide: immobilization of Pd(0) nanoparticles and application in heterogeneous catalysis of cross-coupling reactions.RSC Adv. 2024 Aug 27;14(37):27092-27109. doi: 10.1039/d4ra03401e. eCollection 2024 Aug 22. RSC Adv. 2024. PMID: 39193294 Free PMC article.
-
Recent progress in application of nanocatalysts for carbonylative Suzuki cross-coupling reactions.RSC Adv. 2021 Jan 21;11(4):2112-2125. doi: 10.1039/d0ra09846a. eCollection 2021 Jan 6. RSC Adv. 2021. PMID: 35424173 Free PMC article. Review.
-
Aryl fluorosulfates: powerful and versatile partners in cross-coupling reactions.RSC Adv. 2023 May 3;13(20):13642-13654. doi: 10.1039/d3ra01791e. eCollection 2023 May 2. RSC Adv. 2023. PMID: 37152576 Free PMC article. Review.
References
-
- Wipf P. Tsuchimoto T. Takahashi H. Pure Appl. Chem. 1999;71:415–421.
- Gnanaprakasam B. Milstein D. J. Am. Chem. Soc. 2011;133:1682–1685. doi: 10.1021/ja109944n. - DOI - PubMed
- Takise R. Muto K. Yamaguchi J. Chem. Soc. Rev. 2017;46:5864–5888. doi: 10.1039/C7CS00182G. - DOI - PubMed
- Vessally E. Hosseinian A. Babazadeh M. Edjlali L. Hosseinzadeh-Khanmiri R. Curr. Org. Chem. 2018;22:315–322. doi: 10.2174/1385272821666170619090707. - DOI
-
- Blagosklonny M. V. Fojo T. Int. J. Cancer. 1999;83:151–156. doi: 10.1002/(SICI)1097-0215(19991008)83:2<151::AID-IJC1>3.0.CO;2-5. - DOI - PubMed
- Sohng J. K. Yamaguchi T. Seong C. N. Baik K. S. Park S. C. Lee H. J. Jang S. Y. Simkhada J. R. Yoo J. C. Arch. Pharmacal Res. 2008;31:1339–1345. doi: 10.1007/s12272-001-2115-0. - DOI - PubMed
- Lee D. A. Higginbotham E. J. Am. J. Health-Syst. Pharm. 2005;62:691–699. doi: 10.1093/ajhp/62.7.691. - DOI - PubMed
- Scott L. J. McCormack P. L. Drugs. 2008;68:1239–1272. doi: 10.2165/00003495-200868090-00005. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

