Small molecule PROTACs: an emerging technology for targeted therapy in drug discovery
- PMID: 35519875
- PMCID: PMC9064693
- DOI: 10.1039/c9ra03423d
Small molecule PROTACs: an emerging technology for targeted therapy in drug discovery
Abstract
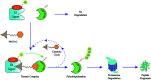
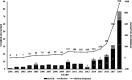
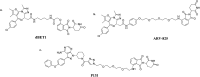
Curing malignant carcinomas is a grand ambition in the development of human health. Over the past decades, targeted therapies have become one of the most successful ways of achieving this. Of these approaches, small molecule inhibitors and monoclonal antibodies are two major methods, however several barriers to their development and clinical use still exist. The use of proteolysis-targeting chimeras (PROTACs) is a new technology through utilizing a intracellular ubiquitin-proteasome system to induce targeted protein degradation, is receiving much attention in the field of targeted therapies. Hetero-bifunctional PROTACs have the potential to eliminate the "undruggable" proteome that comprises about 85% of human proteins, which indicates their great prospects in therapeutic fields. However, there are some hurdles preventing current PROTACs moving from bench to clinic, such as delivery and bioavailability. This review provides an overview of the development of PROTAC technology and will briefly summarize the future possible directions of this approach.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

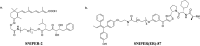


Similar articles
-
PROTACs: An Emerging Targeting Technique for Protein Degradation in Drug Discovery.Bioessays. 2018 Apr;40(4):e1700247. doi: 10.1002/bies.201700247. Epub 2018 Feb 23. Bioessays. 2018. PMID: 29473971 Review.
-
Small-molecule PROTACs: An emerging and promising approach for the development of targeted therapy drugs.EBioMedicine. 2018 Oct;36:553-562. doi: 10.1016/j.ebiom.2018.09.005. Epub 2018 Sep 14. EBioMedicine. 2018. PMID: 30224312 Free PMC article. Review.
-
Targeted protein degradation by PROTACs.Pharmacol Ther. 2017 Jun;174:138-144. doi: 10.1016/j.pharmthera.2017.02.027. Epub 2017 Feb 14. Pharmacol Ther. 2017. PMID: 28223226 Review.
-
Peptide-based PROTACs: Current Challenges and Future Perspectives.Curr Med Chem. 2024;31(2):208-222. doi: 10.2174/0929867330666230130121822. Curr Med Chem. 2024. PMID: 36718000
-
PROTACs technology for targeting non-oncoproteins: Advances and perspectives.Bioorg Chem. 2021 Sep;114:105109. doi: 10.1016/j.bioorg.2021.105109. Epub 2021 Jun 21. Bioorg Chem. 2021. PMID: 34175722 Review.
Cited by
-
Proteolysis-targeting chimeras and their implications in breast cancer.Explor Target Antitumor Ther. 2021;2(6):496-510. doi: 10.37349/etat.2021.00060. Epub 2021 Dec 31. Explor Target Antitumor Ther. 2021. PMID: 36046115 Free PMC article. Review.
-
Native Mass Spectrometry Can Effectively Predict PROTAC Efficacy.ACS Cent Sci. 2020 Jul 22;6(7):1223-1230. doi: 10.1021/acscentsci.0c00049. Epub 2020 Jul 6. ACS Cent Sci. 2020. PMID: 32724856 Free PMC article.
-
Revolutionizing Drug Targeting Strategies: Integrating Artificial Intelligence and Structure-Based Methods in PROTAC Development.Pharmaceuticals (Basel). 2023 Nov 24;16(12):1649. doi: 10.3390/ph16121649. Pharmaceuticals (Basel). 2023. PMID: 38139776 Free PMC article. Review.
-
Prey for the Proteasome: Targeted Protein Degradation-A Medicinal Chemist's Perspective.Angew Chem Int Ed Engl. 2020 Sep 1;59(36):15448-15466. doi: 10.1002/anie.202004310. Epub 2020 Jul 30. Angew Chem Int Ed Engl. 2020. PMID: 32428344 Free PMC article. Review.
-
Proteolysis Targeting Chimera Degraders of the METTL3-14 m6A-RNA Methyltransferase.JACS Au. 2024 Feb 12;4(2):713-729. doi: 10.1021/jacsau.4c00040. eCollection 2024 Feb 26. JACS Au. 2024. PMID: 38425900 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

