Direct synthesis of 2-oxo-acetamidines from methyl ketones, aromatic amines and DMF via copper-catalyzed C(sp3)-H amidination
- PMID: 35519951
- PMCID: PMC9061129
- DOI: 10.1039/c9ra00616h
Direct synthesis of 2-oxo-acetamidines from methyl ketones, aromatic amines and DMF via copper-catalyzed C(sp3)-H amidination
Abstract
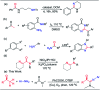
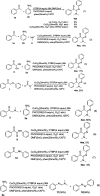
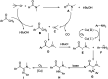
A convenient method for the synthesis of 2-oxo-acetamidines from methyl ketones using aromatic amines and DMF as nitrogen sources is reported via copper-catalyzed C(sp3)-H amidination. Various methyl ketones react readily with aromatic amines and DMF, producing 2-oxo-acetamidines in yields of 47 to 92%. This protocol features the simultaneous formation of C-N and C[double bond, length as m-dash]N bonds using DMF and aromatic amines as two different nitrogen sources. It thus provides an efficient approach to construct acyclic amidines via three C(sp3)-H bond amidination. Based on the preliminary experiments, a plausible mechanism of this transformation is disclosed.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Copper-catalyzed oxidative cross-coupling of α-aminocarbonyl compounds with primary amines toward 2-oxo-acetamidines.Org Biomol Chem. 2017 Oct 4;15(38):8134-8139. doi: 10.1039/c7ob02012k. Org Biomol Chem. 2017. PMID: 28905973
-
Copper-catalyzed aerobic conversion of the C=O bond of ketones to a C≡N bond using ammonium salts as the nitrogen source.Chem Commun (Camb). 2015 Jun 30;51(56):11264-7. doi: 10.1039/c5cc02578h. Chem Commun (Camb). 2015. PMID: 26081463
-
Copper-catalyzed synthesis of α-amino nitriles through methyl transfer from DMF to aromatic amines.Chem Commun (Camb). 2018 Mar 15;54(23):2854-2857. doi: 10.1039/c8cc00485d. Chem Commun (Camb). 2018. PMID: 29457813
-
Metal-catalyzed silylation of sp3C-H bonds.Chem Soc Rev. 2021 Apr 26;50(8):5062-5085. doi: 10.1039/d0cs01392g. Chem Soc Rev. 2021. PMID: 33629997 Review.
-
Recent Advances in Copper-Catalyzed C-N Bond Formation Involving N-Centered Radicals.ChemSusChem. 2021 Dec 17;14(24):5340-5358. doi: 10.1002/cssc.202102243. Epub 2021 Nov 26. ChemSusChem. 2021. PMID: 34750973 Review.
Cited by
-
A Journey from June 2018 to October 2021 with N,N-Dimethylformamide and N,N-Dimethylacetamide as Reactants.Molecules. 2021 Oct 21;26(21):6374. doi: 10.3390/molecules26216374. Molecules. 2021. PMID: 34770783 Free PMC article. Review.
References
-
- Young R. J. Bioorg. Med. Chem. Lett. 2000;10:597. doi: 10.1016/S0960-894X(00)00055-X. - DOI - PubMed
- Stone E. M. Schaller T. H. Bianchi H. Person M. D. Fast W. Biochemistry. 2005;44:13744. doi: 10.1021/bi051341y. - DOI - PubMed
- Doveston R. G. Steendam R. Jones S. Taylor R. J. K. Org. Lett. 2012;14:1122. doi: 10.1021/ol300039x. - DOI - PubMed
- Maccallini C. Patruno A. Besker N. Ali J. Ammazzalorso A. Filippis B. D. Franceschelli S. Giampietro L. Pesce M. Reale M. J. Med. Chem. 2009;52:1481. doi: 10.1021/jm800846u. - DOI - PubMed
- Maccallini C. Patruno A. Lannutti F. Fantacuzzi M. Franceschelli S. Giampietro L. Masella S. Felaco M. Bioorg. Med. Chem. Lett. 2010;20:6495. - PubMed
- Patruno A. Franceschelli S. Pesce M. Maccallini C. Fantacuzzi M. Speranza L. Ferrone A. De Lutiis M. A. Ricciott E. Amoroso R. Biochim. Biophys. Acta. 2012;1820:2095. - PubMed
- Edelmann F. T. Chem. Soc. Rev. 2009;38:2253. doi: 10.1039/B800100F. - DOI - PubMed
- Luo Y. Knuckley B. Lee Y. H. Stallcup M. R. Thompson P. R. J. Am. Chem. Soc. 2006;128:1092. doi: 10.1021/ja0576233. - DOI - PMC - PubMed
- Dai Q. Jiang Y. Yu J.-T. Cheng J. Chem. Commun. 2015;51:16645. doi: 10.1039/C5CC06771E. - DOI - PubMed
-
- Greenhill J. V. Lue P. Prog. Med. Chem. 1993;30:203. doi: 10.1016/S0079-6468(08)70378-3. - DOI - PubMed
- Guile S. D. Alcaraz L. Birkinshaw T. N. Bowers K. C. Ebden M. R. Furber M. Stocks M. J. J. Med. Chem. 2009;52:3123. doi: 10.1021/jm801528x. - DOI - PubMed
- Lee M. Y. Kim M. H. Kim J. Kim S. H. Kim B. T. Jeong I. H. Chang S. Chang S. Y. Bioorg. Med. Chem. Lett. 2010;20:541. doi: 10.1016/j.bmcl.2009.11.104. - DOI - PubMed
- Brasche G. Buchwald S. L. Angew., Chem. Int. Ed. 2008;47:1932. doi: 10.1002/anie.200705420. - DOI - PubMed
- Caron S. Wei L. Douville J. Ghosh A. J. Org. Chem. 2010;75:945. doi: 10.1021/jo902159z. - DOI - PubMed
- Khan R. Arfan M. Mahmood J. Anjum S. Choudhary M. I. Chin. Chem. Lett. 2010;21:905. doi: 10.1016/j.cclet.2010.04.009. - DOI
- Harjani J. R. Liang C. Jessop P. G. J. Org. Chem. 2011;76:1683. doi: 10.1021/jo102358d. - DOI - PubMed
- McGowan M. A. McAvoy C. Z. Buchwald S. L. Org. Lett. 2012;14:3800. doi: 10.1021/ol301700y. - DOI - PMC - PubMed
- Wang Y. F. Zhu X. Chiba S. J. Am. Chem. Soc. 2012;134:3679. doi: 10.1021/ja2120629. - DOI - PubMed
- Taylor J. E. Bull S. D. Williams J. M. J. Chem. Soc. Rev. 2012;41:2109. doi: 10.1039/C2CS15288F. - DOI - PubMed
- Mączka M. Janczak J. Trzebiatowska M. Sieradzki A. Pawlus S. Pikul A. Dalton Trans. 2017;46:8476. doi: 10.1039/C7DT01251A. - DOI - PubMed
- Barker J. Kilner M. Coord. Chem. Rev. 1994;133:219. doi: 10.1016/0010-8545(94)80059-6. - DOI
- Oakley S. H. Soria D. B. Coles M. P. Hitchcock P. B. Dalton Trans. 2004:537. doi: 10.1039/B314707J. - DOI - PubMed
- Edelmann F. T. Adv. Organomet. Chem. 2008;57:183. doi: 10.1039/B314707J. - DOI - PubMed
-
- Mamedov V. A. Zhukova N. A. Syakaev V. V. Gubaidullin A. T. Beschastnova T. N. Adgamova D. I. Samigullina A. I. Latypov S. K. Tetrahedron. 2013;69:1403. doi: 10.1016/j.tet.2012.10.045. - DOI
- Wang D.-H. Yang Y.-X. Yang Y.-Q. Zhao T.-C. Wu X. Wang S.-K. Sci. Bull. 2006;51:785. doi: 10.1007/s11434-006-0785-1. - DOI
- Tantawy A. E Barghash A. Badr S. Gomaa R. Heterocycl. Commun. 2013;19:125.
- Perl N. R. Leighton J. L. Org. Lett. 2007;9:3699. doi: 10.1021/ol701723w. - DOI - PubMed
- Du J.-L. Li L.-J. Li Y.-F. Inorg. Chem. Commun. 2005;8:246. doi: 10.1016/j.inoche.2004.12.021. - DOI
- C Chang H. Son B. C. Song G. Y. Shin J. Y. Ha C. S. Suh H. S. Kim I. Macromol. Res. 2013;21:118. doi: 10.1007/s13233-013-1008-7. - DOI
- Mamedov V. A. Murtazina A. M. Zhukova N. A. Beschastnova T. N. Rizvanov Il. K. Latypov S. K. Tetrahedron. 2014;70:7567. doi: 10.1016/j.tet.2014.07.103. - DOI
-
- Wang B. Du H.-F. Shi Y. A. Angew. Chem. 2008;120:8348. doi: 10.1002/ange.200803184. - DOI - PMC - PubMed
- Neumann J. J. Rakshit S. Droge T. Glorius F. Angew. Chem. 2009;121:7024. doi: 10.1002/ange.200903035. - DOI - PubMed
- Tan Y. Hartwig J. F. J. Am. Chem. Soc. 2010;132:3676. doi: 10.1021/ja100676r. - DOI - PubMed
- Shin K. Kim H. Chang S. Acc. Chem. Res. 2015;48:1040. doi: 10.1021/acs.accounts.5b00020. - DOI - PubMed
- Jiao J. Murakami K. Itami K. ACS Catal. 2016;6:610. doi: 10.1021/acscatal.5b02417. - DOI
- Choi W. Kim J. Ryu T. Kim K.-B. Lee P. H. Org. Lett. 2015;17:3330. - PubMed
- Du C. Li P.-X. Zhu X.-J. Han J.-N. Niu J.-L. Song M.-P. ACS Catal. 2017;7:2810. doi: 10.1021/acscatal.7b00262. - DOI
- Park Y. Kim Y. Chang S. Chem. Rev. 2017;117:9247. doi: 10.1021/acs.chemrev.6b00644. - DOI - PubMed
- Lafollée S. B. Gil R. Prim D. Hannedouche J. Molecules. 2017;22:1901. doi: 10.3390/molecules22111901. - DOI - PMC - PubMed
- Timsina Y. N. Gupton B. F. Ellis K. C. ACS Catal. 2018;8:5732. doi: 10.1021/acscatal.8b01168. - DOI
-
- Rousselet G. Capdeviclle P. Maumy M. Tetrahedron Lett. 1993;34:6395.
- Kukushkin V. Y. Pombeiro A. J. L. Chem. Rev. 2002;102:1771. - PubMed
- Wang J.-F. Xu F. Cai T. Shen Q. Org. Lett. 2008;10:445. doi: 10.1021/ol702739c. - DOI - PubMed
- Ueda S. Nagasawa H. J. Am. Chem. Soc. 2009;131:15080. - PubMed
- Savmarker J. Rydfjord J. Gising J. Odell L. R. Larhed M. Org. Lett. 2012;14:2394. doi: 10.1021/ol300813c. - DOI - PMC - PubMed
- Rydfjord J. Svensson F. Trejos A. Mats Larhed, Chem.–Eur. J. 2013;19:13803. doi: 10.1002/chem.201301809. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources