Simultaneous bifunctional application of solid-state lighting and ratiometric optical thermometer based on double perovskite LiLaMgWO6:Er3+ thermochromic phosphors
- PMID: 35519956
- PMCID: PMC9061103
- DOI: 10.1039/c8ra10242b
Simultaneous bifunctional application of solid-state lighting and ratiometric optical thermometer based on double perovskite LiLaMgWO6:Er3+ thermochromic phosphors
Abstract
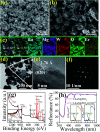
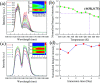
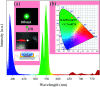
Realization of simultaneous, efficient bifunctional application of thermochromic phosphors on light emitting diodes (LEDs) and as ratiometric thermometers is significant. Herein, doped Er3+ ions are introduced as an activator into double perovskite LiLaMgWO6 host lattice. The developed phosphors can be efficiently excited by a near-ultraviolet LED chip and show bright green emission, mainly at 527 and 543 nm, as well as very low thermal quenching. Their chemical stability is studied, demonstrating excellent application potentials. Furthermore, the temperature sensing properties of LiLaMgWO6:0.01Er3+ were analyzed in the wide range of 303-483 K and show a good exponential relationship between ratiometric intensity and temperature (R 2 > 0.999), as well as high sensitivity (2.24% K-1). Such a system not only optimizes the performance in solid light emitting but also provides an excellent platform for designing high-sensitivity optical thermometry.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
Er3+-Activated NaLaMgWO6 double perovskite phosphors and their bifunctional application in solid-state lighting and non-contact optical thermometry.Dalton Trans. 2019 Mar 26;48(13):4405-4412. doi: 10.1039/c9dt00315k. Dalton Trans. 2019. PMID: 30865208
-
Novel Mn4+-activated LiLaMgWO6 far-red emitting phosphors: high photoluminescence efficiency, good thermal stability, and potential applications in plant cultivation LEDs.RSC Adv. 2018 Jul 30;8(48):27144-27151. doi: 10.1039/c8ra05669b. eCollection 2018 Jul 30. RSC Adv. 2018. PMID: 35539995 Free PMC article.
-
ZnGa2-yAlyO4:Mn2+,Mn4+ Thermochromic Phosphors: Valence State Control and Optical Temperature Sensing.Inorg Chem. 2020 Nov 2;59(21):15969-15976. doi: 10.1021/acs.inorgchem.0c02474. Epub 2020 Oct 15. Inorg Chem. 2020. PMID: 33054208
-
Designing bifunctional platforms for LED devices and luminescence lifetime thermometers: a case of non-rare-earth Mn4+ doped tantalate phosphors.Dalton Trans. 2022 Jun 13;51(23):9062-9071. doi: 10.1039/d2dt01120d. Dalton Trans. 2022. PMID: 35647702
-
Tunable lanthanide/transition metal ion-doped novel phosphors for possible application in w-LEDs: a review.Luminescence. 2020 Feb;35(1):4-33. doi: 10.1002/bio.3712. Epub 2019 Oct 24. Luminescence. 2020. PMID: 31647168 Review.
Cited by
-
Exploration of the Temperature Sensing Ability of La2MgTiO6:Er3+ Double Perovskites Using Thermally Coupled and Uncoupled Energy Levels.Materials (Basel). 2021 Sep 24;14(19):5557. doi: 10.3390/ma14195557. Materials (Basel). 2021. PMID: 34639954 Free PMC article.
References
-
- Chen Y. Wang Y.-C. Zhang Y. Zou H. Lin Z. Zhang G. Zou C. Wang Z. L. Adv. Energy Mater. 2018;8:1802159.
-
- Kong F. Fan X. Kong A. Zhou Z. Zhang X. Shan Y. Adv. Funct. Mater. 2018:1803973.
-
- Marciniak L. Prorok K. Bednarkiewicz A. J. Mater. Chem. C. 2017;5:7890–7897. doi: 10.1039/C7TC02322G. - DOI
-
- McLaurin E. J. Vlaskin V. A. Gamelin D. R. J. Am. Chem. Soc. 2011;133:14978–14980. - PubMed
LinkOut - more resources
Full Text Sources

