A guanosine-based 2-formylphenylborate ester hydrogel with high selectivity to K+ ions
- PMID: 35520041
- PMCID: PMC9055885
- DOI: 10.1039/d0ra05254j
A guanosine-based 2-formylphenylborate ester hydrogel with high selectivity to K+ ions
Abstract
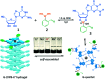
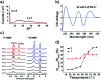
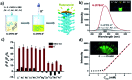
Guanosine-based supramolecular hydrogels are particularly of interest for biomaterial and biomedical purposes, as they are generally biocompatible and stimuli-responsive. We found a strong and long-life transparent hydrogel made by mixing guanosine (G) with 1 equiv. of 2-formylbenzeneboronic acid (2FPB) and KOH. Alkali cations can assist the stacking of individual G-quartet to give extended nanowires, but only K+ ion induces the formation of a stable and self-supporting network hydrogel for a couple of months. Data from variable temperature NMR indicated that guanosine 2-formylbenzeneborate ester and G are the key components of the self-assembly. Further, G-2FPB-K+ hydrogel solution can induce berberine (BBR) fluorescence, showing high selectivity to K+ ion and anti-ion interference capability. A good linear relationship between fluorescent intensity and K+ concentration allowed us to directly detect K+ levels in human blood serum.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Buerkle L. E. Rowan S. J. Chem. Soc. Rev. 2012;41:6089–6102. doi: 10.1039/C2CS35106D. - DOI - PubMed
- Babu S. S. Praveen V. K. Ajayaghosh A. Chem. Rev. 2014;114:1973–2129. doi: 10.1021/cr400195e. - DOI - PubMed
- Weiss R. G. J. Am. Chem. Soc. 2014;136:7519–7530. doi: 10.1021/ja503363v. - DOI - PubMed
- Cheetham A. G. Chakroun R. W. Ma W. Cui H. Chem. Soc. Rev. 2017;46:6638–6663. doi: 10.1039/C7CS00521K. - DOI - PMC - PubMed
-
- Peters G. M. Davis J. T. Chem. Soc. Rev. 2016;45:3188–3206. doi: 10.1039/C6CS00183A. - DOI - PubMed
- Bhattacharyya T. Saha P. Dash J. ACS Omega. 2018;3:2230–2241. doi: 10.1021/acsomega.7b02039. - DOI - PMC - PubMed
- Huppert J. L. Chem. Soc. Rev. 2008;37:1375–1384. doi: 10.1039/B702491F. - DOI - PubMed
- Davis J. T. Spada G. P. Chem. Soc. Rev. 2007;36:296–313. doi: 10.1039/B600282J. - DOI - PubMed
-
- Das R. N. Kumar Y. P. Pagoti S. Patil A. J. Dash J. Chem.–Eur. J. 2012;18:6008–6014. doi: 10.1002/chem.201103814. - DOI - PubMed
- Adhikari B. Shah A. Kraatz H. B. J. Mater. Chem. B. 2014;2:4802–4810. doi: 10.1039/C4TB00702F. - DOI - PubMed
- Li Z. Buerkle L. E. Orseno M. R. Streletzky K. A. Seifert S. Jamieson A. M. Rowan S. J. Langmuir. 2010;26:10093–10101. doi: 10.1021/la100211y. - DOI - PubMed
-
- Peters G. M. Skala L. P. Plank T. N. Hyman B. J. Reddy G. N. M. Marsh A. Brown S. P. Davis J. T. J. Am. Chem. Soc. 2014;136:12596–12599. doi: 10.1021/ja507506c. - DOI - PubMed
- Peters G. M. Skala L. P. Davis J. T. J. Am. Chem. Soc. 2016;138:134–139. doi: 10.1021/jacs.5b08769. - DOI - PubMed
- Plank T. N. Davis J. T. Chem. Commun. 2016;52:5037–5040. doi: 10.1039/C6CC01494A. - DOI - PubMed
- Plank T. N. Skala L. P. Davis J. T. Chem. Commun. 2017;53:6235–6238. doi: 10.1039/C7CC03118A. - DOI - PubMed
- Pieraccini S. Campitiello M. Carducci F. Davis J. T. Mariani P. Masiero S. Org. Biomol. Chem. 2019;17:2759–2769. doi: 10.1039/C9OB00193J. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

