Synthesis of alginate-polycation capsules of different composition: characterization and their adsorption for [As(iii)] and [As(v)] from aqueous solutions
- PMID: 35520048
- PMCID: PMC9055832
- DOI: 10.1039/d0ra05135g
Synthesis of alginate-polycation capsules of different composition: characterization and their adsorption for [As(iii)] and [As(v)] from aqueous solutions
Abstract
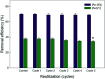
The uptake of arsenite [As(iii)] and arsenate [As(v)] by functionalized calcium alginate (Ca-Alg) beads from aqueous solutions was investigated. Ca-Alg beads were protonated with poly-l-lysine (PLL) or polyethyleneimine (PEI) using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide/N-hydroxysuccinimide (EDC/NHS) or glutaraldehyde (GA) as crosslinking agents. Four types of protonated beads were prepared: Ca-Alg-EDC/NHS (PLL or PEI) and Ca-Alg-GA (PLL or PEI). Fourier transform infrared spectroscopy in total attenuated reflection mode (FTIR-ATR), analysis showed presence and increased intensity of bands corresponding to OH, NH, CH2 and CH3 groups in modifications with both polycations. In addition, thermogravimetric analysis and atomic force microscopy of all modified capsules showed an increase in thermal stability and uniformity of the capsules, respectively. Ca-Alg-EDC/NHS-PLL beads had the maximum adsorption capacity of [As(v)] (312.9 ± 4.7 μg g-1 of the alginate) at pH 7.0 and 15 minute exposure, while Ca-Alg-EDC/NHS-PEI beads had the maximum adsorption capacity of [As(iii)] (1052.1 ± 4.6 μg g-1 of alginate). However, all these EDC containing beads were degraded in the presence of citrate. Ca-Alg-GA-PEI beads removed 252.8 ± 9.7 μg of [As(v)] μg g-1 of alginate and 524.7 ± 5.3 de [As(iii)] μg g-1 of alginate, resulting the most stable capsules and suitable for As removal.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflict of interest to declare.
Figures









References
-
- W. IARC, available from monographs, http://www.iarc.fr/ENG/Classification, 2017
-
- World Health Organization Journal, http://www.who.int/mediacentre/factsheets/fs372/2012
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

