Selective hydrogenation of nitroaromatics to N-arylhydroxylamines in a micropacked bed reactor with passivated catalyst
- PMID: 35520060
- PMCID: PMC9055875
- DOI: 10.1039/d0ra05715k
Selective hydrogenation of nitroaromatics to N-arylhydroxylamines in a micropacked bed reactor with passivated catalyst
Abstract
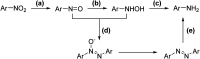
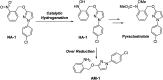
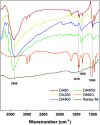
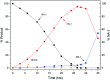
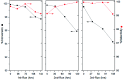
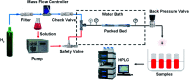
In this contribution, a protocol was established for the selective catalytic hydrogenation of nitroarenes to the corresponding N-arylhydroxylamines. The reduction of 1-(4-chlorophenyl)-3-((2-nitrobenzyl)oxy)-1H-pyrazole, an intermediate in the synthesis of the antifungal reagent pyraclostrobin that includes carbon-chlorine bonds, benzyl groups, carbon-carbon double bonds and other structures that are easily reduced, was chosen as the model reaction for catalyst evaluation and condition optimization. Extensive passivant evaluation showed that RANEY®-nickel treated with ammonia/DMSO (1 : 10, v/v) afforded the optimal result, especially with a particle size of 400-500 mesh. To combine the modified catalyst with continuous-flow reaction technology, the reaction was conducted at room temperature, rendering the desired product with a conversion rate of 99.4% and a selectivity of 99.8%. The regeneration of catalytic activity was also studied, and an in-column strategy was developed by pumping the passivate liquid overnight. Finally, the generality of the method was explored, and 7 substrates were developed, most of which showed a good conversion rate and selectivity, indicating that the method has a certain degree of generality.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- McGill A. D. Zhang W. Wittbrodt J. Wang J. Schlegel H. B. Wang P. G. Bioorg. Med. Chem. 2000;8:405–412. doi: 10.1016/S0968-0896(99)00300-4. - DOI - PubMed
- Yadav J. S. Reddy B. V. S. Sreedhar P. Adv. Synth. Catal. 2003;345:564–567. doi: 10.1002/adsc.200202209. - DOI
- El-Faham A. Albericio F. Eur. J. Org. Chem. 2009;2009:1499–1501. doi: 10.1002/ejoc.200801179. - DOI
- Sabir S. Kumar G. Jat J. L. Org. Biomol. Chem. 2018;16:3314–3327. doi: 10.1039/C8OB00146D. - DOI - PubMed
-
- Karwa S. L. Rajadhyaksha R. A. Ind. Eng. Chem. Res. 1987;26:1746–1750. doi: 10.1021/ie00069a005. - DOI
- Studer M. Neto S. Blaser H. U. Top. Catal. 2000;13:205–212. doi: 10.1023/A:1009050804228. - DOI
- Takenaka Y. Kiyosu T. Choi J.-C. Sakakura T. Yasuda H. Green Chem. 2009;11:1385–1390. doi: 10.1039/B904672K. - DOI
- Takenaka Y. Kiyosu T. Choi J.-C. Sakakura T. Yasuda H. ChemSusChem. 2010;3:1166–1168. doi: 10.1002/cssc.201000137. - DOI - PubMed
- Jawale D. V. Gravel E. Boudet C. Shah N. Geertsen V. Li H. Namboothiri I. N. N. Doris E. Chem. Commun. 2015;51:1739–1742. doi: 10.1039/C4CC09192B. - DOI - PubMed
-
- Cantillo D. Moghaddam M. M. Kappe C. O. J. Org. Chem. 2013;78:4530–4542. doi: 10.1021/jo400556g. - DOI - PubMed
- Plutschack M. B. Pieber B. Gilmore K. Seeberger P. H. Chem. Rev. 2017;117:11796–11893. doi: 10.1021/acs.chemrev.7b00183. - DOI - PubMed
- Britton J. Majumdar S. Weiss G. A. Chem. Soc. Rev. 2018;47:5891–5918. doi: 10.1039/C7CS00906B. - DOI - PMC - PubMed
- Akwi F. M. Watts P. Chem. Commun. 2018;54:13894–13928. doi: 10.1039/C8CC07427E. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

