Synthesis of benzonaphthofuroquinones and benzoylnaphthindolizinediones by reactions of flavonoids with dichlone under basylous, oxygenous and aqueous conditions: their cytotoxic and apoptotic activities
- PMID: 35520063
- PMCID: PMC9055798
- DOI: 10.1039/d0ra06043g
Synthesis of benzonaphthofuroquinones and benzoylnaphthindolizinediones by reactions of flavonoids with dichlone under basylous, oxygenous and aqueous conditions: their cytotoxic and apoptotic activities
Abstract
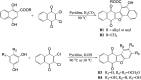
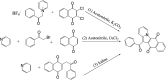
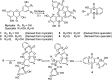
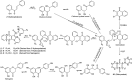
Using flavonoids and dichlone as substrates, benzonaphthofuroquinones (1, 2, 3, 5, 6, novel; 4 new) and benzoylnaphthindolizinediones (7, 8, known; 9, new) were synthesized through common base-catalyzed method and a new method of combining base-catalyzed with O2/H2O exposing. The possible reaction mechanisms may involve the process like isomerization, hydration, oxidation, decomposition and intermolecular condensation. Benzonaphthofuroquinones (2, 3, 4, 5) were found to exhibit potent cytotoxicity against carcinoma cell lines and low toxicity to normal cell lines. The compounds 4 and 5 not only expressed a significant late-stage-apoptosis against human leukemia and melanoma, but also promoted the cleavage of caspase-3 and PARP in human leukemia, which suggested that the late-stage-apoptosis and caspase-3 pathway may be responsible for the cytotoxicities of these benzonaphthofuroquinones. The replacement of the furan ring with pyrrole system in benzoylnaphthindolizinediones (7, 8, 9) resulted in the loss of anticancer activity.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
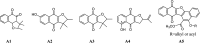
References
-
- Morello A. Pavani M. Garbarino J.-A. Chamy M.-C. Frey C. Mancilla J. Guerrero A. Repetto Y. Ferreira J. Comp. Biochem. Physiol., Part C: Pharmacol., Toxicol. Endocrinol. 1995;112:119–128. - PubMed
-
- Cho H. Park S.-J. Lee K.-I. Bull. Korean Chem. Soc. 2005;26:1829–1832. doi: 10.5012/bkcs.2005.26.11.1829. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

