Preparation and characterisation of PHT-loaded chitosan lecithin nanoparticles for intranasal drug delivery to the brain
- PMID: 35520085
- PMCID: PMC9055806
- DOI: 10.1039/d0ra04890a
Preparation and characterisation of PHT-loaded chitosan lecithin nanoparticles for intranasal drug delivery to the brain
Abstract
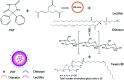
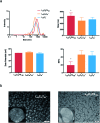
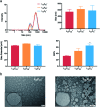
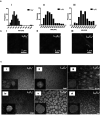
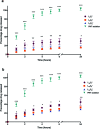
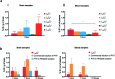
The use of nanoparticles (NPs) for intranasal (IN) drug delivery to the brain represents a hopeful strategy to enhance brain targeting of anti-epileptic drugs. In the present work, chitosan-lecithin NPs loaded with phenytoin (PHT), were prepared using the nano-precipitation method. The spherical nature of the NPs and their stability were confirmed using scanning and transmission electron microscopy, while the average dynamic size and zeta potential were measured using dynamic light scattering. The encapsulation efficiency of PHT was higher than 60% for all prepared NPs. Release studies showed that the amount of released PHT was directly related to the amount of chitosan used. The optimum preparation, L10Ci + was administered via the IN route, and the levels of PHT in the brain were measured in three-time points. Two experimental controls were given via the intraperitoneal (IP) and IN routes. The highest PHT amount reaching 1.01 ± 0.55% for L10Ci +, which was associated with a sustained release of PHT. These preliminary findings show that the IN delivery of PHT-loaded NPs is very promising for managing epilepsy. The direct nose-to-brain approach increases the safety margins of PHT, while the sustained release could improve patient compliance in a clinical setting.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Nordli D. R., Focal and Multifocal Seizures, Swaiman's Pediatric Neurology, Elsevier, 2017, pp. 531–537
LinkOut - more resources
Full Text Sources

