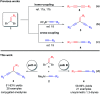
Highly selective cross-coupling reactions of 1,1-dibromoethylenes with alkynylaluminums for the synthesis of aryl substituted conjugated enediynes and unsymmetrical 1,3-diynes
- PMID: 35520111
- PMCID: PMC9062886
- DOI: 10.1039/d2ra02127g
Highly selective cross-coupling reactions of 1,1-dibromoethylenes with alkynylaluminums for the synthesis of aryl substituted conjugated enediynes and unsymmetrical 1,3-diynes
Abstract
A highly efficient method for the synthesis of aryl substituted conjugated enediynes and unsymmetrical 1,3-diynes via selective cross-coupling reactions of 1,1-dibromoethylenes with alkynylaluminums using the Pd(OAc)2-DPPE and Pd2(dba)3-TFP complexes as catalysts, respectively, has been successfully developed. Though the alkyl substituted conjugated enediynes and unsymmetrical 1,3-diynes were not obtained, this case is also remarkable as the same starting materials could selectively produce either aryl substituted conjugated enediynes or unsymmetrical 1,3-diynes in moderate to excellent yields (up to 99%) in the different Pd-phosphine catalytic systems.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Gold-catalyzed oxidative cross-coupling of terminal alkynes: selective synthesis of unsymmetrical 1,3-diynes.J Am Chem Soc. 2014 Sep 24;136(38):13174-7. doi: 10.1021/ja5078365. Epub 2014 Sep 12. J Am Chem Soc. 2014. PMID: 25184690
-
Highly regio- and stereoselective synthesis of boron-substituted enynes via copper-catalyzed borylation of conjugated diynes.Org Lett. 2015 Feb 20;17(4):860-3. doi: 10.1021/ol503720w. Epub 2015 Feb 9. Org Lett. 2015. PMID: 25665129
-
Cobalt-Catalyzed Cross-Benzannulation of Conjugated Enynes and Diynes.J Org Chem. 2015 Jul 17;80(14):7311-6. doi: 10.1021/acs.joc.5b01198. Epub 2015 Jul 2. J Org Chem. 2015. PMID: 26084604
-
Highly Selective Room-Temperature Suzuki-Miyaura Coupling of Bromo-2-sulfonyloxypyridines for Unsymmetrical Diarylpyridines.J Org Chem. 2020 Jun 5;85(11):7399-7412. doi: 10.1021/acs.joc.0c00793. Epub 2020 May 15. J Org Chem. 2020. PMID: 32370500
-
Copper Catalyzed C-H Activation.Chem Rec. 2019 Jul;19(7):1302-1318. doi: 10.1002/tcr.201800107. Epub 2018 Oct 30. Chem Rec. 2019. PMID: 30375153 Review.
References
-
- Nicolaou K. C. Dai M. W. Tsay S. C. Estevez V. A. Wrasidlo W. Science. 1992;256:1172. - PubMed
- Lin Y. Y. Wang Y. J. Chen J. H. Lee C. F. Synlett. 2012;23:930.
- Sandrine V. Etienne D. Muriel A. Corinne A. Marc P. Adv. Synth. Catal. 2013;355:2584.
- Fong H. K. H. Brunel J. M. Longeon A. Bourguet-Kondracki M.-L. Barker D. Copp B. R. Org. Biomol. Chem. 2017;15:6194. - PubMed
- Qian D. Zhang J. Acc. Chem. Res. 2020;53:2358. - PubMed
- Campeau D. Rayo D. F. L. Mansour A. Muratov K. Gagosz F. Chem. Rev. 2021;121:8756. - PubMed
- Zhao T. Pu X. Han W. Gao G. Org. Lett. 2021;23:1199. - PubMed
-
- Liu J. Z. Lam J. W. Y. Tang B. Z. Chem. Rev. 2009;109:5799. - PubMed
- Shi W. Lei A. Tetrahedron Lett. 2014;55:2763.
- Matsuda Y. Naoe S. Oishi S. Fujii N. Ohno H. Chem.–Eur. J. 2015;21:1463. - PubMed
- Lampkowski J. S. Uthappa D. M. Halonski J. F. Maza J. C. Young D. D. J. Org. Chem. 2016;81:12520. - PMC - PubMed
- Sang H. L. Wu C. Phua G. G. D. Ge S. ACS Catal. 2019;9:10109.
- Dhananjaya K. Nagaraju V. Raghuram G. Arup K. K. Subhashree N. Chandi C. M. Tetrahedron Lett. 2020;61:151775.
- Sebastian M. W. Gerhard H. Front. Chem. 2021;9:635826. - PubMed
-
- Shi Shun A. L. K. Tykwinski R. R. Angew. Chem., Int. Ed. 2006;45:1034. - PubMed
-
- Cao H.-Y. Guo X.-F. Zhu X.-F. Li S.-S. Zhen Y.-S. Oncol. Rep. 2017;37:3329. - PubMed
-
- Gholami M. Tykwinski R. R. Chem. Rev. 2006;106:4997. - PubMed
LinkOut - more resources
Full Text Sources