Hydroxyapatite formation in biomimetic synthesis with the interface of a pDA@SIS membrane
- PMID: 35520114
- PMCID: PMC9064378
- DOI: 10.1039/d2ra00910b
Hydroxyapatite formation in biomimetic synthesis with the interface of a pDA@SIS membrane
Abstract
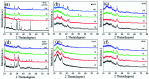
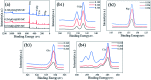
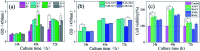
Porcine decellularized small intestine submucosa (SIS) is a collagen membrane, which offers great potential as an organic substrate template in mineralization processes due to its good biodegradability and biocompatibility. However, a long period of mineralization and low efficiency are apparent, and the mechanism of collagen fiber mineralization has often been neglected in the previous literature. Thus, in this paper, we present a novel model of biomimetic collagen mineralization which uses dopamine (DA) molecules with the activating and retouching function of SIS collagen membranes and regulating collagen mineralization to construct the structure of mineralized collagen hard tissues. The crystal biomimetic mineralization growth of calcium phosphate on membranes is studied in different solid-liquid interfaces with a double ion self-assembled diffusion system under the simulated physiological microenvironment. In the system, pDA@SIS membranes are used to control the concentration of Ca2+ and PO4 3- ionic diffusion to generate supersaturation reaction conditions in 1-14 days. The system can successfully obtain polycrystals with low crystallinity on the pDA-collagen complex template surface of collagen fibers and along the collagen fibers. It initiates a generalized bionic mineralization pathway which can reduce the nucleation interfacial energy to promote rapid hydroxyapatite (HAP) nucleation and crystallization and accelerate the rate of collagen fiber mineralization. The pDA@SIS mineralized collagen membrane shows good biocompatibility with 100% cellular activity in the CCK-8 test, which significantly improved the adhesion proliferation of MC3T3-E1 cells. The pDA-SIS collagen complex, as a new type of mineralization template, may propose a new collagen mineralization strategy to produce a mineralized pDA@SIS scaffold bone-like material for tissue engineering or can potentially be applied in bone repair and regeneration.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures







Similar articles
-
A Dopamine Acrylamide Molecule for Promoting Collagen Biomimetic Mineralization and Regulating Crystal Growth Direction.ACS Appl Mater Interfaces. 2021 Aug 25;13(33):39142-39156. doi: 10.1021/acsami.1c12412. Epub 2021 Aug 16. ACS Appl Mater Interfaces. 2021. PMID: 34433244
-
[Effects of different polymers on biomimetic mineralization of small intestine submucosal scaffolds].Beijing Da Xue Xue Bao Yi Xue Ban. 2024 Feb 18;56(1):17-24. doi: 10.19723/j.issn.1671-167X.2024.01.004. Beijing Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 38318891 Free PMC article. Chinese.
-
c-Axis-Oriented Platelets of Crystalline Hydroxyapatite in Biomimetic Intrafibrillar Mineralization of Polydopamine-Functionalized Collagen Type I.ACS Omega. 2022 Feb 7;7(6):4821-4831. doi: 10.1021/acsomega.1c05198. eCollection 2022 Feb 15. ACS Omega. 2022. PMID: 35187302 Free PMC article.
-
Periosteum and development of the tissue-engineered periosteum for guided bone regeneration.J Orthop Translat. 2022 Feb 16;33:41-54. doi: 10.1016/j.jot.2022.01.002. eCollection 2022 Mar. J Orthop Translat. 2022. PMID: 35228996 Free PMC article. Review.
-
Surface modification of small intestine submucosa in tissue engineering.Regen Biomater. 2020 Aug;7(4):339-348. doi: 10.1093/rb/rbaa014. Epub 2020 May 18. Regen Biomater. 2020. PMID: 32793379 Free PMC article. Review.
Cited by
-
Application of biphasic mineralized collagen/polycaprolactone scaffolds in the repair of large load-bearing bone defects: A study in a sheep model.J Orthop Translat. 2025 Apr 21;52:138-149. doi: 10.1016/j.jot.2025.03.014. eCollection 2025 May. J Orthop Translat. 2025. PMID: 40322042 Free PMC article.
-
Evaluation of enamel remineralization potential and anticariogenic efficacy of polydopamine coated biogenic amorphous calcium phosphate.Clin Oral Investig. 2025 May 20;29(6):302. doi: 10.1007/s00784-025-06384-4. Clin Oral Investig. 2025. PMID: 40389610
References
-
- Bradt J. H. Mertig M. Teresiak A. Pompe W. Biomimetic mineralization of collagen by combined fibril assembly and calcium phosphate formation. Chem. Mater. 1999;11:2694–2701. doi: 10.1021/cm991002p. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

