Enhancement of protein stability by an additional disulfide bond designed in human neuroglobin
- PMID: 35520156
- PMCID: PMC9062612
- DOI: 10.1039/c8ra10390a
Enhancement of protein stability by an additional disulfide bond designed in human neuroglobin
Abstract
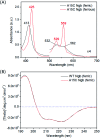
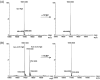
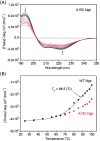
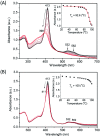
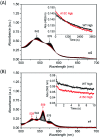
Human neuroglobin (Ngb) forms an intramolecular disulfide bond between Cys46 and Cys55, with a third Cys120 near the protein surface, which is a promising protein model for heme protein design. In order to protect the free Cys120 and to enhance the protein stability, we herein developed a strategy by designing an additional disulfide bond between Cys120 and Cys15 via A15C mutation. The design was supported by molecular modeling, and the formation of Cys15-Cys120 disulfide bond was confirmed experimentally by ESI-MS analysis. Molecular modeling, UV-Vis and CD spectroscopy showed that the additional disulfide bond caused minimal structural alterations of Ngb. Meanwhile, the disulfide bond of Cys15-Cys120 was found to enhance both Gdn·HCl-induced unfolding stability (increased by ∼0.64 M) and pH-induced unfolding stability (decreased by ∼0.69 pH unit), as compared to those of WT Ngb with a single native disulfide bond of Cys46-Cys55. Moreover, the half denaturation temperature (T m) of A15C Ngb was determined to be higher than 100 °C. In addition, the disulfide bond of Cys15-Cys120 has slight effects on protein function, such as an increase in the rate of O2 release by ∼1.4-fold. This study not only suggests a crucial role of the artificial disulfide in protein stabilization, but also lays the groundwork for further investigation of the structure and function of Ngb, as well as for the design of other functional heme proteins, based on the scaffold of A15C Ngb with an enhanced stability.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
LinkOut - more resources
Full Text Sources

