Synthesis and physico-chemical properties of a H-cardanol triazole zinc porphyrin conjugate
- PMID: 35520169
- PMCID: PMC9060588
- DOI: 10.1039/c8ra09998g
Synthesis and physico-chemical properties of a H-cardanol triazole zinc porphyrin conjugate
Abstract
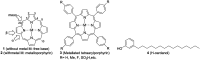
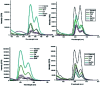
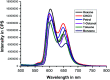
Although a large number of natural and non-natural metalloporphyrins are known, examples with fluorescence and fat-soluble properties are rare. We have achieved the synthesis of a fluorescent and fat-soluble zinc porphyrin incorporating four units of hydrogenated cardanol (H-cardanol). The synthesis is sustainable since the product is derived from cashew-nut shell liquid (CNSL), which is a renewable and bio-waste material. The H-cardanol triazole zinc porphyrin conjugate (HTZPC) was synthesized through applying a copper(i) catalyzed azide-alkyne cycloaddition (CuAAC) reaction between a H-cardanol derived azide and a tetraarylporphyrin derived alkyne. The absorption and emission properties of the hydrocarbon solvent soluble HTZPC were evaluated using UV-vis and fluorescence emission spectra obtained in various solvents. The results were compared with related molecules like a triazole-zinc porphyrin conjugate (TZPC), zinc tetra-C(4)-methoxyphenyl porphyrin (ZP), and a H-cardanol-triazole conjugate (HTC). The results showed that HTZPC undergoes J-type aggregation in both non-polar and highly polar solvents, which is dictated by van der Waals attractive forces between H-cardanol units in polar solvents (e.g. methanol and dimethylformamide) and π-π stacking interactions between porphyrin units in non-polar solvents (hexane). Moreover, the spectra indicated that the triazole units could stabilize the zinc porphyrin via intermolecular coordinate-complex formation. We anticipate that fat-soluble HTZPC could find applications in medical fields (e.g. in the photodynamic therapy of fat tissue).
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures








References
-
- Milgrom L. R., The Colors of Life: An Introduction to the Chemistry of Porphyrins and Related Compounds, Oxford University Press, Oxford, 1997
-
- Kadish K. M., Porphyrin Science: With Applications to Chemistry, Physics, Materials Science, Engineering, Biology and Medicine, World Scientific, 2010
-
- Ogoshi H. Mizutani T. Acc. Chem. Res. 1998;31:81–89. doi: 10.1021/ar9603165. - DOI
-
- Di Natale C. Martinelli E. Magna G. Mandoj F. Monti D. Nardis S. Stefanelli M. Paolesse R. J. Porphyrins Phthalocyanines. 2017;21:769–781. doi: 10.1142/S1088424617300026. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

