Direct production of olefins via syngas conversion over Co2C-based catalyst in slurry bed reactor
- PMID: 35520170
- PMCID: PMC9060519
- DOI: 10.1039/c8ra10477h
Direct production of olefins via syngas conversion over Co2C-based catalyst in slurry bed reactor
Abstract
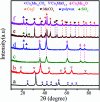
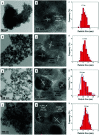
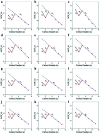
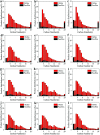
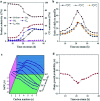
Direct production of olefins via syngas conversion over a Co2C-based catalyst was investigated in a slurry bed reactor (SBR). It was found that the total selectivities to olefins and oxygenates reached 88.8C% at a CO conversion of 29.5% at 250 °C, 5 bar and H2/CO = 0.5. The hydrocarbon distribution greatly deviated from the classical Anderson-Schulz-Flory (ASF) distribution, with only 2.6C% methane selectivity was obtained. XRD and TEM characterization verified that the Co2C nanoprisms with special exposed facts of (101) and (020) constitutes the Fischer-Tropsch to olefins (FTO) active site. The catalytic activity increased gradually with rising the reaction temperature, while the product distribution almost kept unchanged under various reaction condition in SBR. Compared to the reaction in FBR, the Co2C-based catalyst exhibited relative better catalytic performance during FTO process in SBR. Specifically, a higher CO conversion, a lower methane selectivity and a higher total selectivities to olefins and oxygenates were achieved in SBR. In addition, the catalyst can be in situ reduced in slurry bed reactor at mild temperature (300 °C) and no obvious deactivation was found within nearly 100 h time-on-stream, which suggested a promising route for the direct production of olefins via syngas in industrial application.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Galvis H. M. T. Jong K. P. D. ACS Catal. 2013;3:2130–2149. doi: 10.1021/cs4003436. - DOI
-
- Chen W. Lin T. Dai Y. An Y. Yu F. Zhong L. Li S. Sun Y. Catal. Today. 2018;311:8–22. doi: 10.1016/j.cattod.2017.09.019. - DOI
-
- An Y. Lin T. Yu F. Yang Y. Zhong L. Wu M. Sun Y. Sci. China: Chem. 2017;60:887–903. doi: 10.1007/s11426-016-0464-1. - DOI
-
- Xiao K. Bao Z. Qi X. Wang X. Zhong L. Fang K. Lin M. Sun Y. Chin. J. Catal. 2013;34:116–129. doi: 10.1016/S1872-2067(11)60496-8. - DOI
-
- Dai Y. Yu F. Li Z. An Y. Lin T. Yang Y. Zhong L. Wang H. Sun Y. Chin. J. Chem. 2017;35:918–926. doi: 10.1002/cjoc.201600748. - DOI
LinkOut - more resources
Full Text Sources

