Electrochemical performance of myoglobin based on TiO2-doped carbon nanofiber decorated electrode and its applications in biosensing
- PMID: 35520203
- PMCID: PMC9060622
- DOI: 10.1039/c8ra07910b
Electrochemical performance of myoglobin based on TiO2-doped carbon nanofiber decorated electrode and its applications in biosensing
Abstract
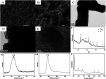
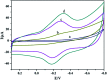
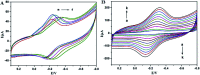
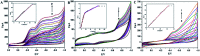
A new biosensing strategy based on a TiO2-doped carbon nanofiber (CNF) composite modified electrode was developed. TiO2@CNF was prepared by electrospinning with further carbonization, before being characterized by various methods and used for electrode modification on the surface of carbon ionic liquid electrode (CILE). Myoglobin (Mb) was further immobilized on the modified electrode surface. The results of ultraviolet-visible (UV-vis) and Fourier transform infrared (FT-IR) spectroscopy showed that Mb maintained its native structure without denaturation in the composite film. Direct electron transfer and the electrocatalytic properties of Mb on the electrode surface were further investigated. A pair of quasi-reversible redox peaks appeared on the cyclic voltammogram, indicating that direct electrochemistry of Mb was realized in the nanocomposite film. This could be attributed to the specific properties of TiO2@CNF nanocomposite, including a large surface-to-volume ratio, good biocompatibility and high conductivity. Nafion/Mb/TiO2@CNF/CILE exhibited an excellent electrochemical catalytic ability in the reduction of trichloroacetic acid, NaNO2 and H2O2. All results demonstrated potential applications of TiO2@CNF in third-generation electrochemical biosensors.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Li J. H. Kuang D. Z. Feng Y. L. Liu M. Q. Voltammetric determination of o-phenylenediamine in industrial wastewater by an electrode modified with nanocomposite film of TiO2/hydroxyapatite/MWCNT. Chin. J. Anal. Chem. 2011;39:1864–1870.
-
- Hassen D. Shenashen M. A. El-Safty S. A. Selim M. M. Isago H. Elmarakbi A. El-Safty A. Yamaguchi H. Nitrogen-doped carbon-embedded TiO2 nanofibers as promising oxygen reduction reaction electrocatalysts. J. Power Sources. 2016;330:292–303. doi: 10.1016/j.jpowsour.2016.08.140. - DOI
-
- Zhang Z. H. Zhang S. He L. H. Peng D. L. Yan F. F. Wang M. H. Zhao J. H. Zhang H. Z. Fang S. M. Feasible electrochemical biosensor based on plasma polymerization-assisted composite of polyacrylic acid and hollow TiO2 spheres for sensitively detecting lysozyme. Biosens. Bioelectron. 2015;74:384–390. doi: 10.1016/j.bios.2015.06.062. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

