Different diameters of titanium dioxide nanotubes modulate Saos-2 osteoblast-like cell adhesion and osteogenic differentiation and nanomechanical properties of the surface
- PMID: 35520235
- PMCID: PMC9062999
- DOI: 10.1039/c9ra00761j
Different diameters of titanium dioxide nanotubes modulate Saos-2 osteoblast-like cell adhesion and osteogenic differentiation and nanomechanical properties of the surface
Abstract
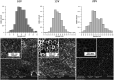
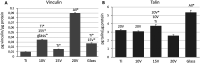
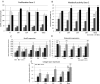
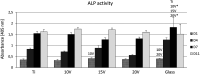
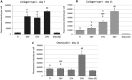
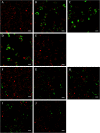
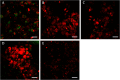
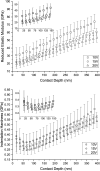
The formation of nanostructures on titanium implant surfaces is a promising strategy to modulate cell adhesion and differentiation, which are crucial for future application in bone regeneration. The aim of this study was to investigate how the nanotube diameter and/or nanomechanical properties alter human osteoblast like cell (Saos-2) adhesion, growth and osteogenic differentiation in vitro. Nanotubes, with diameters ranging from 24 to 66 nm, were fabricated on a commercially pure titanium (cpTi) surface using anodic oxidation with selected end potentials of 10 V, 15 V and 20 V. The cell response was studied in vitro on untreated and nanostructured samples using a measurement of metabolic activity, cell proliferation, alkaline phosphatase activity and qRT-PCR, which was used for the evaluation of osteogenic marker expression (collagen type I, osteocalcin, RunX2). Early cell adhesion was investigated using SEM and ELISA. Adhesive molecules (vinculin, talin), collagen and osteocalcin were also visualized using confocal microscopy. Moreover, the reduced elastic modulus and indentation hardness of nanotubes were assessed using a TriboIndenter™. Smooth and nanostructured cpTi both supported cell adhesion, proliferation and bone-specific mRNA expression. The nanotubes enhanced collagen type I and osteocalcin synthesis, compared to untreated cpTi, and the highest synthesis was observed on samples modified with 20 V nanotubes. Significant differences were found in the cell adhesion, where the vinculin and talin showed a dot-like distribution. Both the lowest reduced elastic modulus and indentation hardness were assessed from 20 V samples. The nanotubes of mainly 20 V samples showed a high potential for their use in bone implantation.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
In vitro evaluation of a novel nanostructured Ti-36Nb-6Ta alloy for orthopedic applications.Nanomedicine (Lond). 2020 Aug;15(19):1843-1859. doi: 10.2217/nnm-2020-0123. Epub 2020 Aug 5. Nanomedicine (Lond). 2020. PMID: 32752935
-
The diameter of nanotubes formed on Ti-6Al-4V alloy controls the adhesion and differentiation of Saos-2 cells.Int J Nanomedicine. 2015 Nov 20;10:7145-63. doi: 10.2147/IJN.S87474. eCollection 2015. Int J Nanomedicine. 2015. PMID: 26648719 Free PMC article.
-
Cell interaction with modified nanotubes formed on titanium alloy Ti-6Al-4V.Mater Sci Eng C Mater Biol Appl. 2016 Aug 1;65:313-22. doi: 10.1016/j.msec.2016.04.037. Epub 2016 Apr 13. Mater Sci Eng C Mater Biol Appl. 2016. PMID: 27157757
-
Differential expression of osteoblast-like cells on self-organized titanium dioxide nanotubes.J Dent Sci. 2024 Dec;19(Suppl 1):S26-S37. doi: 10.1016/j.jds.2024.09.001. Epub 2024 Sep 16. J Dent Sci. 2024. PMID: 39807437 Free PMC article.
-
Improved osteoblast adhesion and osseointegration on TiO2 nanotubes surface with hydroxyapatite coating.Dent Mater J. 2019 Mar 31;38(2):278-286. doi: 10.4012/dmj.2018-118. Epub 2018 Dec 11. Dent Mater J. 2019. PMID: 30541994
Cited by
-
Insight into antibacterial effect of titanium nanotubular surfaces with focus on Staphylococcus aureus and Pseudomonas aeruginosa.Sci Rep. 2024 Jul 27;14(1):17303. doi: 10.1038/s41598-024-68266-1. Sci Rep. 2024. PMID: 39068252 Free PMC article.
-
Creating an extracellular matrix-like three-dimension structure to enhance the corrosion resistance and biological responses of titanium implants.J Dent Sci. 2024 Dec;19(Suppl 1):S70-S80. doi: 10.1016/j.jds.2024.09.007. Epub 2024 Sep 24. J Dent Sci. 2024. PMID: 39807433 Free PMC article.
-
Recovering Osteoblast Functionality on TiO2 Nanotube Surfaces Under Diabetic Conditions.Int J Nanomedicine. 2022 Nov 18;17:5469-5488. doi: 10.2147/IJN.S387386. eCollection 2022. Int J Nanomedicine. 2022. PMID: 36426372 Free PMC article.
-
Mesenchymal stem cell interaction with Ti6Al4V alloy pre-exposed to simulated body fluid.RSC Adv. 2020 Feb 13;10(12):6858-6872. doi: 10.1039/c9ra08912h. eCollection 2020 Feb 13. RSC Adv. 2020. PMID: 35493900 Free PMC article.
-
The Deposition of a Lectin from Oreochromis niloticus on the Surface of Titanium Dioxide Nanotubes Improved the Cell Adhesion, Proliferation, and Osteogenic Activity of Osteoblast-like Cells.Biomolecules. 2021 Nov 24;11(12):1748. doi: 10.3390/biom11121748. Biomolecules. 2021. PMID: 34944393 Free PMC article.
References
-
- Okabe T. Hero H. Cells Mater. 1995;5:211–230.
-
- Bjursten L. M. Rasmusson L. Oh S. Smith G. C. Brammer K. S. Jin S. J. Biomed. Mater. Res., Part A. 2010;92A:1218–1224. - PubMed
LinkOut - more resources
Full Text Sources

