Indirect Comparisons via Sorafenib for the Comparative Effectiveness of Two PD-1/PD-L1 Inhibitors to Treat Advanced Hepatocellular Carcinoma Patients without Prior Systemic Therapies
- PMID: 35520275
- PMCID: PMC9064682
- DOI: 10.2147/CLEP.S352045
Indirect Comparisons via Sorafenib for the Comparative Effectiveness of Two PD-1/PD-L1 Inhibitors to Treat Advanced Hepatocellular Carcinoma Patients without Prior Systemic Therapies
Abstract
Introduction: Advanced hepatocellular carcinoma (HCC) represents a major public health threat. Several emerging combination therapies have shown promising results for the first-line treatment of advanced HCC. The present study compared the efficacy of atezolizumab plus bevacizumab (AB) with lenvatinib plus pembrolizumab (LP), which were two of the leading combination therapies.
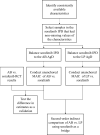
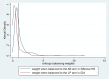
Methods: The outcomes of the present analysis were overall survival (OS) time and progression-free survival (PFS) time. Two matching-adjusted indirect comparisons (MAICs) were first conducted using the individual-level patient data (IPD) of the sorafenib arm from a previous clinical trial and the aggregate data (AgD) of the AB and LP arms from the corresponding published trials. From the MAICs, the hazard ratios (HRs) of AB and LP vs sorafenib were estimated by conducting weighted Cox regressions. The HRs from the two MAICs were then pooled to conduct a second-order indirect comparison of AB vs LP.
Results: In the MAIC analyses, AB had better efficacy on both OS (HR: 0.58, 95% CI: 0.42-0.79) and PFS (HR: 0.59, 95% CI: 0.47-0.76) than sorafenib, whereas LP had significantly better efficacy on PFS (HR: 0.62, 95% CI: 0.41-0.94) but not OS (HR: 0.83, 95% CI: 0.52-1.32). In the second-order comparison, AB was insignificantly more efficacious on OS (HR: 0.71, 95% CI: 0.42-1.23) than and similarly efficacious on PFS (HR: 0.95, 95% CI: 0.60-1.51) as the LP regimen.
Conclusion: LP regimen may be a potential first-line immunotherapy option for advanced HCC given its comparative effectiveness in relation to AB.
Keywords: PD-1; PD-L1; adjusted; balancing; first-line; matching; overall; progression-free; survival; unresectable.
© 2022 Jiang et al.
Conflict of interest statement
The authors claim no conflicts of interest related to the subject of the submitted work.
Figures
Similar articles
-
Comparative Efficacy of Cabozantinib and Regorafenib for Advanced Hepatocellular Carcinoma.Adv Ther. 2020 Jun;37(6):2678-2695. doi: 10.1007/s12325-020-01378-y. Epub 2020 May 18. Adv Ther. 2020. PMID: 32424805 Free PMC article.
-
Transarterial Radioembolization Versus Atezolizumab-Bevacizumab in Unresectable Hepatocellular Carcinoma: A Matching-Adjusted Indirect Comparison of Time to Deterioration in Quality of Life.Adv Ther. 2022 May;39(5):2035-2051. doi: 10.1007/s12325-022-02099-0. Epub 2022 Mar 12. Adv Ther. 2022. PMID: 35279814 Free PMC article.
-
Pembrolizumab+chemotherapy versus atezolizumab+chemotherapy+/-bevacizumab for the first-line treatment of non-squamous NSCLC: A matching-adjusted indirect comparison.Lung Cancer. 2021 May;155:175-182. doi: 10.1016/j.lungcan.2021.03.020. Epub 2021 Mar 30. Lung Cancer. 2021. PMID: 33839603 Clinical Trial.
-
Comparison of Efficacy of Systemic Therapies in Advanced Hepatocellular Carcinoma: Updated Systematic Review and Frequentist Network Meta-Analysis of Randomized Controlled Trials.J Hepatocell Carcinoma. 2021 Mar 22;8:145-154. doi: 10.2147/JHC.S268305. eCollection 2021. J Hepatocell Carcinoma. 2021. PMID: 33791250 Free PMC article. Review.
-
Pembrolizumab for Previously Treated Advanced or Metastatic Urothelial Cancer: An Evidence Review Group Perspective of a NICE Single Technology Appraisal.Pharmacoeconomics. 2019 Jan;37(1):19-27. doi: 10.1007/s40273-018-0689-3. Pharmacoeconomics. 2019. PMID: 30030817 Review.
Cited by
-
FDA-Approved Monoclonal Antibodies for Unresectable Hepatocellular Carcinoma: What Do We Know So Far?Int J Mol Sci. 2023 Jan 31;24(3):2685. doi: 10.3390/ijms24032685. Int J Mol Sci. 2023. PMID: 36769004 Free PMC article. Review.
-
Safety and Efficacy of Atezolizumab and Bevacizumab Combination as a First Line Treatment of Advanced Hepatocellular Carcinoma.J Hepatocell Carcinoma. 2023 Oct 2;10:1689-1708. doi: 10.2147/JHC.S347932. eCollection 2023. J Hepatocell Carcinoma. 2023. PMID: 37808223 Free PMC article. Review.
-
Impact of baseline body mass index on the long-term prognosis of advanced hepatocellular carcinoma treated with immunotherapy.World J Gastroenterol. 2024 Oct 7;30(37):4132-4148. doi: 10.3748/wjg.v30.i37.4132. World J Gastroenterol. 2024. PMID: 39474397 Free PMC article.
-
A process to validate prognostic factors for unanchored matching-adjusted indirect comparison of single-arm trials in oncology: a proof-of-concept study.J Comp Eff Res. 2025 May;14(5):e240235. doi: 10.57264/cer-2024-0235. Epub 2025 Apr 7. J Comp Eff Res. 2025. PMID: 40190245 Free PMC article.
-
Impact of baseline hepatitis B virus viral load on the long-term prognosis of advanced hepatocellular carcinoma treated with immunotherapy.World J Gastrointest Oncol. 2024 Jun 15;16(6):2504-2519. doi: 10.4251/wjgo.v16.i6.2504. World J Gastrointest Oncol. 2024. PMID: 38994160 Free PMC article.
References
-
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatol. 2018;68(2):723–750. - PubMed
-
- Tang H, Huang Y, Duan W, Li C, Meng X, Dong J. A concise review of current guidelines for the clinical management of hepatocellular carcinoma in Asia. Transl Cancer Res. 2017;6(6):1214–1225.
LinkOut - more resources
Full Text Sources
Research Materials

