Synthesis and characterization of a supported Pd complex on volcanic pumice laminates textured by cellulose for facilitating Suzuki-Miyaura cross-coupling reactions
- PMID: 35520332
- PMCID: PMC9054627
- DOI: 10.1039/d0ra04521g
Synthesis and characterization of a supported Pd complex on volcanic pumice laminates textured by cellulose for facilitating Suzuki-Miyaura cross-coupling reactions
Retraction in
-
Retraction: Synthesis and characterization of a supported Pd complex on volcanic pumice laminates textured by cellulose for facilitating Suzuki-Miyaura cross-coupling reactions.RSC Adv. 2024 Apr 17;14(18):12463. doi: 10.1039/d4ra90042a. eCollection 2024 Apr 16. RSC Adv. 2024. PMID: 38633495 Free PMC article.
Abstract
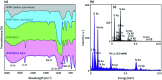
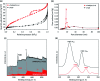
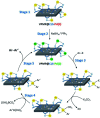
Herein, a novel high-performance heterogeneous catalytic system made of volcanic pumice magnetic particles (VPMP), cellulose (CLS) natural polymeric texture, and palladium nanoparticles (Pd NPs) is presented. The introduced VPMP@CLS-Pd composite has been designed based on the principles of green chemistry, and suitably applied in the Suzuki-Miyaura cross-coupling reactions, as an efficient heterogeneous catalytic system. Concisely, the inherent magnetic property of VPMP (30 emu g-1) provides a great possibility for separation of the catalyst particles from the reaction mixture with great ease. In addition, high heterogeneity and high structural stability are obtained by this composition resulting in remarkable recyclability (ten times successive use). As the main catalytic sites, palladium nanoparticles (Pd NPs) are finely distributed onto the VPMP@CLS structure. To catalyze the Suzuki-Miyaura cross-coupling reactions producing biphenyl pharmaceutical derivatives, the present Pd NPs were reduced from chemical state Pd2+ to Pd0. In this regard, a plausible mechanism is submitted in the context as well. As the main result of the performed analytical methods (including FT-IR, EDX, VSM, TGA, FESEM, TEM, BTE, and XPS), it is shown that the spherical-shaped nanoscale Pd particles have been well distributed onto the surfaces of the porous laminate-shaped VPMP. However, the novel designed VPMP@CLS-Pd catalyst is used for facilitating the synthetic reactions of biphenyls, and high reaction yields (∼98%) are obtained in a short reaction time (10 min) by using a small amount of catalytic system (0.01 g), under mild conditions (room temperature).
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
Authors declare no conflict of interest.
Figures





References
-
- Xue X. Zhaoa Z. Wang Y. Retraction: a miraculous chiral Ir–Rh bimetallic nanocatalyst for asymmetric hydrogenation of activated ketones. Org. Chem. Front. 2019;6:3603. doi: 10.1039/C9QO90089F. - DOI
-
- Qiu Y. Zhang Y. Jin L. Pan L. Du G. Ye D. Wang D. Immobilization of manganese dioxide nanoparticles on modified poly 2,4-dichlorostyrene microspheres: a highly efficient and recyclable catalyst for borrowing hydrogen reactions. Org. Chem. Front. 2019;6:3420–3427. doi: 10.1039/C9QO00892F. - DOI
-
- Bao G. Bai J. Li C. Synergistic effect of the Pd–Ni bimetal/carbon nanofiber composite catalyst in Suzuki coupling reaction. Org. Chem. Front. 2019;6:352–361. doi: 10.1039/C8QO01100A. - DOI
-
- Gawande M. B. Monga Y. Zboril R. Sharma R. K. Silica-decorated magnetic nanocomposites for catalytic applications. Coord. Chem. Rev. 2015;288:118–143. doi: 10.1016/j.ccr.2015.01.001. - DOI
-
- Maleki A. Taheri-Ledari R. Soroushnejad M. Surface functionalization of magnetic nanoparticles via palladium-catalyzed Diels-Alder approach. ChemistrySelect. 2018;3:13057–13062. doi: 10.1002/slct.201803001. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

