RhoA-ROCK competes with YAP to regulate amoeboid breast cancer cell migration in response to lymphatic-like flow
- PMID: 35520391
- PMCID: PMC9065582
- DOI: 10.1096/fba.2021-00055
RhoA-ROCK competes with YAP to regulate amoeboid breast cancer cell migration in response to lymphatic-like flow
Abstract
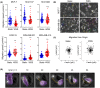
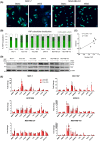
Lymphatic drainage generates force that induces prostate cancer cell motility via activation of Yes-associated protein (YAP), but whether this response to fluid force is conserved across cancer types is unclear. Here, we show that shear stress corresponding to fluid flow in the initial lymphatics modifies taxis in breast cancer, whereas some cell lines use rapid amoeboid migration behavior in response to fluid flow, a separate subset decrease movement. Positive responders displayed transcriptional profiles characteristic of an amoeboid cell state, which is typical of cells advancing at the edges of neoplastic tumors. Regulation of the HIPPO tumor suppressor pathway and YAP activity also differed between breast subsets and prostate cancer. Although subcellular localization of YAP to the nucleus positively correlated with overall velocity of locomotion, YAP gain- and loss-of-function demonstrates that YAP inhibits breast cancer motility but is outcompeted by other pro-taxis mediators in the context of flow. Specifically, we show that RhoA dictates response to flow. GTPase activity of RhoA, but not Rac1 or Cdc42 Rho family GTPases, is elevated in cells that positively respond to flow and is unchanged in cells that decelerate under flow. Disruption of RhoA or the RhoA effector, Rho-associated kinase (ROCK), blocked shear stress-induced motility. Collectively, these findings identify biomechanical force as a regulator amoeboid cell migration and demonstrate stratification of breast cancer subsets by flow-sensing mechanotransduction pathways.
Keywords: ECM–receptor interactions; actomyosin cytoskeleton; biomechanical force; hippo; mechanotransduction; motility; shear stress.
©2022 The Authors. FASEB BioAdvances published by The Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors have declared explicitly that there are no conflicts of interest in connection with this article.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
