Recent application of visible-light induced radicals in C-S bond formation
- PMID: 35520400
- PMCID: PMC9054237
- DOI: 10.1039/d0ra03086d
Recent application of visible-light induced radicals in C-S bond formation
Retraction in
-
Retraction: Recent application of visible-light induced radicals in C-S bond formation.RSC Adv. 2024 Sep 5;14(39):28345. doi: 10.1039/d4ra90096k. eCollection 2024 Sep 4. RSC Adv. 2024. PMID: 39239283 Free PMC article.
Abstract
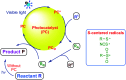
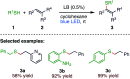
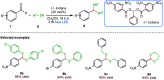
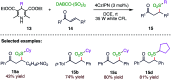
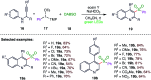
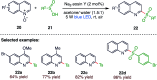
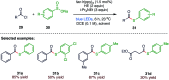
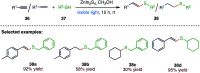
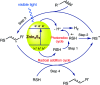
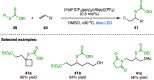
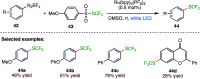
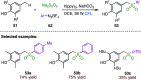
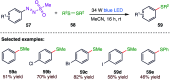
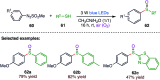
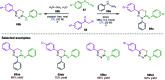
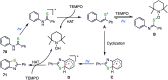
The sulphur centered radicals, produced from various organic compounds, in high efficiency by single-electron-transfer (SET) oxidation. These radicals are highly reactive intermediates having various applications in the construction of organosulphur compounds in the field of synthetic organic chemistry. These S-centred radical-mediated organic transformations have been achieved using photoredox catalysts, including organic dyes and transition metal catalysts, as well as in the absence of any catalyst. Compared with previous methods, photoredox catalysis is inexpensive and features the advantages of being environmentally benign, highly efficient and easy to use. This review focuses on recent developments in the photocatalyzed carbon-sulphur bond formation.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


















Similar articles
-
Generation of alkyl and acyl radicals by visible-light photoredox catalysis: direct activation of C-O bonds in organic transformations.Beilstein J Org Chem. 2024 Jun 14;20:1348-1375. doi: 10.3762/bjoc.20.119. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 38887583 Free PMC article. Review.
-
Synthetic Utilization of α-Aminoalkyl Radicals and Related Species in Visible Light Photoredox Catalysis.Acc Chem Res. 2016 Sep 20;49(9):1946-56. doi: 10.1021/acs.accounts.6b00251. Epub 2016 Aug 9. Acc Chem Res. 2016. PMID: 27505299
-
When Light Meets Nitrogen-Centered Radicals: From Reagents to Catalysts.Acc Chem Res. 2020 May 19;53(5):1066-1083. doi: 10.1021/acs.accounts.0c00090. Epub 2020 Apr 14. Acc Chem Res. 2020. PMID: 32286794
-
Synthetic and Mechanistic Implications of Chlorine Photoelimination in Nickel/Photoredox C(sp3)-H Cross-Coupling.Acc Chem Res. 2021 Feb 16;54(4):988-1000. doi: 10.1021/acs.accounts.0c00694. Epub 2021 Jan 29. Acc Chem Res. 2021. PMID: 33511841 Free PMC article.
-
Redox-neutral carbon-heteroatom bond formation under photoredox catalysis.Chem Commun (Camb). 2023 Jun 6;59(46):7004-7027. doi: 10.1039/d3cc01873c. Chem Commun (Camb). 2023. PMID: 37171250 Review.
Cited by
-
Generation of alkyl and acyl radicals by visible-light photoredox catalysis: direct activation of C-O bonds in organic transformations.Beilstein J Org Chem. 2024 Jun 14;20:1348-1375. doi: 10.3762/bjoc.20.119. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 38887583 Free PMC article. Review.
-
Visible-Light-Triggered and Rhodamine B-Catalyzed Thiolation of Bromoalkynes: Accessing Alkynyl Thioethers.J Org Chem. 2025 Aug 1;90(30):10880-10891. doi: 10.1021/acs.joc.5c01295. Epub 2025 Jul 23. J Org Chem. 2025. PMID: 40700612 Free PMC article.
-
Recent advances in visible-light graphitic carbon nitride (g-C3N4) photocatalysts for chemical transformations.RSC Adv. 2022 Jun 21;12(28):18245-18265. doi: 10.1039/d2ra01797k. eCollection 2022 Jun 14. RSC Adv. 2022. Retraction in: RSC Adv. 2024 Sep 5;14(39):28346. doi: 10.1039/d4ra90095b. PMID: 35800311 Free PMC article. Retracted. Review.
-
3DPAFIPN as a halogenated dicyanobenzene-based photosensitizer catalyzed gram-scale photosynthesis of pyrano[2,3-d]pyrimidine scaffolds.Sci Rep. 2023 Aug 12;13(1):13142. doi: 10.1038/s41598-023-40360-w. Sci Rep. 2023. PMID: 37573466 Free PMC article.
-
Visible-light acridinium-based organophotoredox catalysis in late-stage synthetic applications.RSC Adv. 2023 Apr 6;13(16):10958-10986. doi: 10.1039/d3ra01364b. eCollection 2023 Apr 3. RSC Adv. 2023. PMID: 37033422 Free PMC article. Review.
References
-
- Shen C. Zhang P. Sun Q. Bai S. Andy Hor T. S. Liu X. Chem. Soc. Rev. 2015;44:291. doi: 10.1039/C4CS00239C. - DOI - PubMed
- Xia C. Wei Z. Shen C. Xu J. Yang Y. Su W. Zhang P. RSC Adv. 2015;65:52588. doi: 10.1039/C5RA06474K. - DOI
- Lu Q. Zhang J. Wei F. Qi Y. Wang H. Liu Z. Lei A. Angew. Chem. Int. Ed. 2013;52(28):7156. doi: 10.1002/anie.201301634. - DOI - PubMed
- Xu J. Shen C. Zhu X. Zhang P. Ajitha M. J. Huang K. W. An Z. Liu X. Chem.–Asian J. 2016;11:882. doi: 10.1002/asia.201501407. - DOI - PubMed
- Rocke B. N. Bahnck K. B. Herr M. Lavergne S. Mascitti V. Perreault C. Polivkova J. Shavnya A. Org. Lett. 2014;16:154. doi: 10.1021/ol4031233. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

