Non-covalent interactions between sertraline stereoisomers and 2-hydroxypropyl-β-cyclodextrin: a quantum chemistry analysis
- PMID: 35520401
- PMCID: PMC9054229
- DOI: 10.1039/c9ra10218c
Non-covalent interactions between sertraline stereoisomers and 2-hydroxypropyl-β-cyclodextrin: a quantum chemistry analysis
Abstract
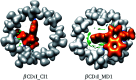
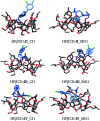
Inclusion compounds formed between sertraline stereoisomers and β-cyclodextrin, and 2-hydroxypropyl-β-cyclodextrin, were analyzed by using quantum chemistry methods. The exploration of the potential energy surface was performed using chemical intuition and classical molecular mechanics. This approach delivered around 200 candidates for low energy adducts, which were optimized through the PBE0/6-31G(d,p) method, and after this process solvent effects were considered by the continuous solvent model. This analysis showed that β-cyclodextrin and 2-hydroxypropyl-β-cyclodextrin are good trappers of sertraline, although the isomers suggested by molecular dynamics presented higher binding energies than those obtained by chemical intuition. The role of hydrogen bonds in the formation of adducts was studied using the non-covalent interactions index and the quantum theory of atoms in molecules. In this article we concluded that these interactions are present in all adducts, however, they are not important in the stabilization of these inclusion compounds. The molecular electrostatic potential indicates that Coulomb interactions could be responsible for the formation of these systems, although sophisticated solvent models must be used to confirm this conclusion, which are impractical in this case because of the sizes involved in these systems.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







Similar articles
-
Inclusion complex thermodynamics: The β-cyclodextrin and sertraline complex example.J Mol Graph Model. 2015 Nov;62:11-17. doi: 10.1016/j.jmgm.2015.08.008. Epub 2015 Aug 22. J Mol Graph Model. 2015. PMID: 26340534
-
Stereoselective separation of (1S, 4S)-sertraline from medicinal reaction mixtures by countercurrent chromatography with hydroxypropyl-β-cyclodextrin as stereoselective selector.J Sep Sci. 2019 Aug;42(16):2734-2742. doi: 10.1002/jssc.201900340. Epub 2019 Jun 24. J Sep Sci. 2019. PMID: 31207064
-
Multimodal molecular encapsulation of nicardipine hydrochloride by beta-cyclodextrin, hydroxypropyl-beta-cyclodextrin and triacetyl-beta-cyclodextrin in solution. Structural studies by 1H NMR and ROESY experiments.Eur J Pharm Sci. 2003 Apr;18(5):285-96. doi: 10.1016/s0928-0987(03)00025-3. Eur J Pharm Sci. 2003. PMID: 12694880
-
Empirical, thermodynamic and quantum-chemical investigations of inclusion complexation between flavanones and (2-hydroxypropyl)-cyclodextrins.Food Chem. 2012 Sep 15;134(2):926-32. doi: 10.1016/j.foodchem.2012.02.207. Epub 2012 Mar 8. Food Chem. 2012. PMID: 23107709
-
Computational tools to study non-covalent interactions and confinement effects in chemical systems.Chem Commun (Camb). 2024 Mar 12;60(22):3008-3018. doi: 10.1039/d3cc06347j. Chem Commun (Camb). 2024. PMID: 38376468 Review.
Cited by
-
Application of Molecular Dynamics Simulations in the Analysis of Cyclodextrin Complexes.Int J Mol Sci. 2021 Aug 30;22(17):9422. doi: 10.3390/ijms22179422. Int J Mol Sci. 2021. PMID: 34502331 Free PMC article. Review.
-
Thermodynamic Studies of Interactions between Sertraline Hydrochloride and Randomly Methylated β-Cyclodextrin Molecules Supported by Circular Dichroism Spectroscopy and Molecular Docking Results.Int J Mol Sci. 2021 Nov 16;22(22):12357. doi: 10.3390/ijms222212357. Int J Mol Sci. 2021. PMID: 34830239 Free PMC article.
References
-
- Fineberg N. A. Baldwin D. S. Drummond L. M. Wyatt S. Hanson J. Gopi S. Kaur S. Reid J. Marwah V. Sachdev R. A. Pampaloni I. Shahper S. Varlakova Y. Mpavaenda D. Manson C. O’Leary C. Irvine K. Monji-Patel D. Shodunke A. Dyer T. Dymond A. Barton G. Wellsted D. Int. Clin. Psychopharmacol. 2018;33:334–348. doi: 10.1097/YIC.0000000000000237. - DOI - PMC - PubMed
-
- Al-Nimry S. S. Jaber M. A. Lat. Am. J. Pharm. 2017;36:665–672.
LinkOut - more resources
Full Text Sources

