A physical approach for the estimation of the SERS enhancement factor through the enrichment and separation of target molecules using magnetic adsorbents
- PMID: 35520413
- PMCID: PMC9054121
- DOI: 10.1039/d0ra03019h
A physical approach for the estimation of the SERS enhancement factor through the enrichment and separation of target molecules using magnetic adsorbents
Abstract
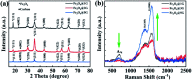
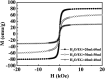
The controllable synthesis of nanosized Fe3O4 (10-20 nm) encapsulated in different numbers of graphene layers (1-5 layers) (Fe3O4@DGL NPs) was realized through a facile and green hydrothermal reaction at a temperature as low as 200 °C. The competitive reduction-oxidation between reducing ethylene glycol (EG) and oxidizing H2O under hydrothermal conditions resulted in the emergence of a magnetic Fe3O4 core. Then, the pyrolytic reaction of the polyvinyl alcohol (PVA) molecules attached to the surface of the Fe3O4 core with different surface densities led to the formation of graphene with a controlled number of layers. These Fe3O4@DGL NPs exhibited fast adsorption and sensitive SERS detection for rhodamine B (RhB). A physical and mathematical model was proposed for the estimation of the enhancement factor (EF) by combining the adsorption efficiency and SERS of RhB. This approach and model are applicable for the adsorption, sensitive SERS detection and determination of SERS EF when using functional magnetic nanoparticles as the adsorbent. The Fe3O4@1G NPs were also used as a novel nano-adsorbent for the fast removal of Escherichia coli (E. coli) from an aqueous solution. The Fe3O4@1G NPs regenerated after 3 cycles also showed high efficiency in the adsorption and separation of RhB and E. coli.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Similar articles
-
Core-shell Fe3O4 polydopamine nanoparticles serve multipurpose as drug carrier, catalyst support and carbon adsorbent.ACS Appl Mater Interfaces. 2013 Sep 25;5(18):9167-71. doi: 10.1021/am402585y. Epub 2013 Sep 6. ACS Appl Mater Interfaces. 2013. PMID: 24010676
-
Novel magnetically separable anhydride-functionalized Fe3O4@SiO2@PEI-NTDA nanoparticles as effective adsorbents: synthesis, stability and recyclable adsorption performance for heavy metal ions.RSC Adv. 2019 Mar 26;9(17):9533-9545. doi: 10.1039/c8ra10310k. eCollection 2019 Mar 22. RSC Adv. 2019. PMID: 35520722 Free PMC article.
-
Graphene Oxide/Polyvinyl Alcohol/Fe3O4 Nanocomposite: An Efficient Adsorbent for Co(II) Ion Removal.J Anal Methods Chem. 2021 Mar 9;2021:6670913. doi: 10.1155/2021/6670913. eCollection 2021. J Anal Methods Chem. 2021. PMID: 33763287 Free PMC article.
-
Removal of antibiotics from aqueous solution by using magnetic Fe3O4/red mud-nanoparticles.Sci Total Environ. 2019 Jun 20;670:539-546. doi: 10.1016/j.scitotenv.2019.03.205. Epub 2019 Mar 15. Sci Total Environ. 2019. PMID: 30909031
-
Facile synthesis of Au nanoparticle-coated Fe3O4 magnetic composite nanospheres and their application in SERS detection of malachite green.Spectrochim Acta A Mol Biomol Spectrosc. 2020 Nov 5;241:118532. doi: 10.1016/j.saa.2020.118532. Epub 2020 May 26. Spectrochim Acta A Mol Biomol Spectrosc. 2020. PMID: 32610212
Cited by
-
Multifunctional Hollow Porous Fe3O4@N-C Nanocomposites as Anodes of Lithium-Ion Battery, Adsorbents and Surface-Enhanced Raman Scattering Substrates.Molecules. 2023 Jul 3;28(13):5183. doi: 10.3390/molecules28135183. Molecules. 2023. PMID: 37446845 Free PMC article.
References
-
- Arora S. Rajpal A. Kazmi A. A. Antimicrobial Activity of Bacterial Community for Removal of Pathogens during Vermifiltration. J. Environ. Eng. 2016;142:04016012. doi: 10.1061/(ASCE)EE.1943-7870.0001080. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

