A quick and versatile one step metal-organic chemical deposition method for supported Pt and Pt-alloy catalysts
- PMID: 35520426
- PMCID: PMC9054134
- DOI: 10.1039/d0ra03001e
A quick and versatile one step metal-organic chemical deposition method for supported Pt and Pt-alloy catalysts
Abstract
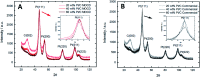
A simple, modified Metal-Organic Chemical Deposition (MOCD) method for Pt, PtRu and PtCo nanoparticle deposition onto a variety of support materials, including C, SiC, B4C, LaB6, TiB2, TiN and a ceramic/carbon nanofiber, is described. Pt deposition using Pt(acac)2 as a precursor is shown to occur via a mixed solid/liquid/vapour precursor phase which results in a high Pt yield of 90-92% on the support material. Pt and Pt alloy nanoparticles range 1.5-6.2 nm, and are well dispersed on all support materials, in a one-step method, with a total catalyst preparation time of ∼10 hours (2.4-4× quicker than conventional methods). The MOCD preparation method includes moderate temperatures of 350 °C in a tubular furnace with an inert gas supply at 2 bar, a high pressure (2-4 bar) compared to typical MOCVD methods (∼0.02-10 mbar). Pt/C catalysts with Pt loadings of 20, 40 and 60 wt% were synthesised, physically characterised, electrochemically characterised and compared to commercial Pt/C catalysts. TEM, XRD and ex situ EXAFS show similar Pt particle sizes and Pt particle shape identifiers, namely the ratio of the third to first Pt coordination numbers modelled from ex situ EXAFS, between the MOCD prepared catalysts and commercial catalysts. Moreover, electrochemical characterisation of the Pt/C MOCD catalysts obtained ORR mass activities with a maximum of 428 A gPt -1 at 0.9 V, which has similar mass activities to the commercial catalysts (80-160% compared to the commercial Pt/C catalysts).
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
-
- Zhang J., PEM Fuel Cell Electrocatalysts and Catalyst Layers: Fundamentals and Applications, Springer London, 2008
-
- Pollet B. G. Kocha S. S. Staffell I. Current status of automotive fuel cells for sustainable transport. Curr. Opin. Electrochem. 2019;16:90–95. doi: 10.1016/j.coelec.2019.04.021. - DOI
-
- Durst J. Simon C. Siebel A. Rheinländer P. J. Schuler T. Hanzlik M. Herranz J. Hasché F. Gasteiger H. A. Hydrogen Oxidation and Evolution Reaction (HOR/HER) on Pt Electrodes in Acid vs. Alkaline Electrolytes: Mechanism, Activity and Particle Size Effects. ECS Trans. 2014;64:1069–1080. doi: 10.1149/06403.1069ecst. - DOI
-
- Antolini E. Salgado J. R. C. Gonzalez E. R. The stability of Pt–M (M = first row transition metal) alloy catalysts and its effect on the activity in low temperature fuel cells: a literature review and tests on a Pt–Co catalyst. J. Power Sources. 2006;160:957–968. doi: 10.1016/j.jpowsour.2006.03.006. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

