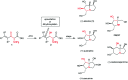
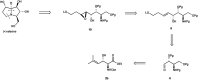
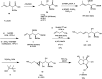
Enantioselective synthesis of anti-3-alkenyl-2-amido-3-hydroxy esters: application to the total synthesis of (+)-alexine
- PMID: 35520501
- PMCID: PMC9059938
- DOI: 10.1039/c9ra00173e
Enantioselective synthesis of anti-3-alkenyl-2-amido-3-hydroxy esters: application to the total synthesis of (+)-alexine
Abstract
A straightforward synthesis of anti-3-alkenyl-2-amido-3-hydroxy esters from the corresponding racemic α-amino-β-keto esters by using a ATH/DKR protocol has been developed. This method gives moderate to excellent yields with high chemo-, diastereo- and enantioselectivities for a broad range of substrates. In order to highlight the versatility of the methodology it was applied in an efficient asymmetric synthesis of the polyhydroxylated pyrrolizidine alkaloid (+)-alexine.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Miller M. J. Acc. Chem. Res. 1986;19:49–56. doi: 10.1021/ar00122a004. - DOI
- Pansare S. V. Vederas J. C. J. Org. Chem. 1987;52:4804–4810. doi: 10.1021/jo00230a033. - DOI
- Lotz B. T. Miller M. J. J. Org. Chem. 1993;58:618–625. doi: 10.1021/jo00055a013. - DOI
- Tanner D. Angew. Chem., Int. Ed. Engl. 1994;33:599–619. doi: 10.1002/anie.199405991. - DOI
- Ford P. W. Gustafson K. R. McKee T. C. Shigematsu N. Maurizi L. K. Pannell L. K. Williams D. E. Dilip de Silva E. Lassota P. Allen T. M. Van Soest R. Andersen R. J. Boyd M. R. J. Am. Chem. Soc. 1999;121:5899–5909. doi: 10.1021/ja990582o. - DOI
- Nicolaou K. C. Boddy C. N. C. Bräse S. Winssinger N. Angew. Chem., Int. Ed. 1999;38:2096–2152. doi: 10.1002/(SICI)1521-3773(19990802)38:15<2096::AID-ANIE2096>3.0.CO;2-F. - DOI - PubMed
- Misner J. W. Fisher J. W. Gardner J. P. Pedersen S. W. Trinkle K. L. Jackson B. G. Zhang T. Y. Tetrahedron Lett. 2003;44:5991–5993. doi: 10.1016/S0040-4039(03)01483-7. - DOI
- Wen S.-J. Yao Z.-J. Org. Lett. 2004;6:2721–2724. doi: 10.1021/ol049065n. - DOI - PubMed
- Nicolaou K. C. Dethe D. H. Leung G. Y. C. Zou B. Chen D. Y. K. Chem.–Asian J. 2008;3:413–429. doi: 10.1002/asia.200700361. - DOI - PubMed
- Echeverria P.-G. Prévost S. Cornil J. Férard C. Reymond S. Guérinot A. Cossy J. Ratovelomanana-Vidal V. Phansavath P. Org. Lett. 2014;16:2390–2393. doi: 10.1021/ol500720j. - DOI - PubMed
-
- He L. Byun H.-S. Bittman R. J. Org. Chem. 2000;65:7627–7633. doi: 10.1021/jo001226n. - DOI - PubMed
- Bodkin J. A. Bacskay G. B. McLeod M. D. Org. Biomol. Chem. 2008;6:2544–2553. doi: 10.1039/B803310B. - DOI - PubMed
- Patel J. Clavé G. Renard P. Y. Franck X. Angew. Chem., Int. Ed. 2008;47:4224–4227. doi: 10.1002/anie.200800860. - DOI - PubMed
- Donohoe T. J. Callens C. K. A. Flores A. Lacy A. R. Rathi A. H. Chem.–Eur. J. 2011;17:58–76. doi: 10.1002/chem.201002323. - DOI - PubMed
- Masruri A. C. W. McLeod M. D. J. Org. Chem. 2012;77:8480–8491. doi: 10.1021/jo301372y. - DOI - PubMed
- Singjunla Y. Baudoux J. Rouden J. Org. Lett. 2013;15:5770–5773. doi: 10.1021/ol402805f. - DOI - PubMed
- Chaumont P. Baudoux J. Maddaluno J. Rouden J. Harrison-Marchand A. J. Org. Chem. 2018;83:8081–8091. doi: 10.1021/acs.joc.8b00901. - DOI - PubMed
-
- Kobayashi J. Nakamura M. Mori Y. Yamashita Y. Kobayashi S. J. Am. Chem. Soc. 2004;126:9192–9193. doi: 10.1021/ja047597t. - DOI - PubMed
- Willis M. C. Cutting G. A. Piccio V. J. D. Durbin M. J. John M. P. Angew. Chem., Int. Ed. 2005;44:1543–1545. doi: 10.1002/anie.200462125. - DOI - PubMed
- Maeda T. Makino K. Iwasaki M. Hamada Y. Chem.–Eur. J. 2010;16:11954–11962. doi: 10.1002/chem.201001298. - DOI - PubMed
- Zheng Y. Deng L. Chem. Sci. 2015;6:6510–6514. doi: 10.1039/C5SC02116B. - DOI - PMC - PubMed
-
- Joergensen K. A. Chem. Rev. 1989;89:431–458. doi: 10.1021/cr00093a001. - DOI
- Adam W. Peters K. Renz M. Angew. Chem., Int. Ed. Engl. 1994;33:1107–1108. doi: 10.1002/anie.199411071. - DOI
- Yang D. Wong M.-K. Yip Y.-C. J. Org. Chem. 1995;60:3887–3889. doi: 10.1021/jo00117a046. - DOI
- Adam W. Mitchell C. M. Angew. Chem., Int. Ed. Engl. 1996;35:533–535. doi: 10.1002/anie.199605331. - DOI
- Kamata K. Yamaguchi K. Hikichi S. Mizuno N. Adv. Synth. Catal. 2003;345:1193–1196. doi: 10.1002/adsc.200303123. - DOI
- Lindstrom U. M. Ding R. Hidestal O. Chem. Commun. 2005:1773–1774. doi: 10.1039/B500190K. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources