Synthesis and biological evaluation of 2,2-dimethylbenzopyran derivatives as potent neuroprotection agents
- PMID: 35520520
- PMCID: PMC9059924
- DOI: 10.1039/c8ra10424g
Synthesis and biological evaluation of 2,2-dimethylbenzopyran derivatives as potent neuroprotection agents
Abstract
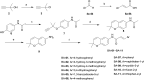
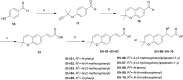
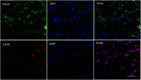
The development of novel neuroprotection agents is of great significance for the treatment of ischemic stroke. In this study, a series of compounds comprising 2,2-dimethylbenzopyran groups and cinnamic acid groups have been synthesized. Preferential combination principles and bioisostere that improved the neuroprotective effect of the compounds were identified for this series via biological activity assay in vitro. Meanwhile, a functional reversal group of the acrylamide amide resulted in the most active compounds. Among them, BN-07 significantly improved the morphology of neurons and obviously increased cell survival rate of primary neurons induced by oxygen glucose deprivation (OGD), superior to clinically used anti-ischemic stroke drug edaravone (Eda). Overall, our findings may provide an alternative strategy for the design of novel anti-ischemic stroke agents with more potency than Eda.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Go A. S. Mozaffarian D. Roger V. L. Benjamin E. J. Berry J. D. Borden W. B. Bravata D. M. Dai S. Ford E. S. Fox C. S. Franco S. Fullerton H. J. Gillespie C. Hailpern S. M. Heit J. A. Howard V. J. Huffman M. D. Kissela B. M. Kittner S. J. Lackland D. T. Lichtman J. H. Lisabeth L. D. Magid D. Marcus G. M. Marelli A. Matchar D. B. McGuire D. K. Mohler E. R. Moy C. S. Mussolino M. E. Nichol G. Paynter N. P. Schreiner P. J. Sorlie P. D. Stein J. Turan T. N. Virani S. S. Wong N. D. Woo D. Turner M. B. Circulation. 2013;127:e6–e245. - PMC - PubMed
-
- Kilic I. D. Hakeem A. Marmagkiolis K. Paixao A. Grunwald I. Mutlu D. Abou-Sherif S. Gundogdu B. Kulaksizoglu S. Ates I. Wholey M. Goktekin O. Cilingiroglu M. Cardiovasc. Revasc. Med. 2018 doi: 10.1016/j.carrev.2018.07.010. - DOI
LinkOut - more resources
Full Text Sources

