Preparation and application of solid-state upconversion materials based on sodium polyacrylate
- PMID: 35520550
- PMCID: PMC9064687
- DOI: 10.1039/c9ra01027k
Preparation and application of solid-state upconversion materials based on sodium polyacrylate
Abstract
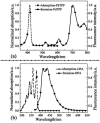
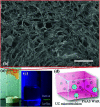
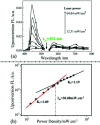
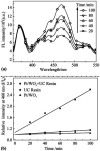
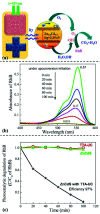
By loading a microemulsion containing both sensitizer and emitter into porous sodium polyacrylate (PAAS), a water-absorbent resin (WAR) upconversion (UC) material was fabricated for photocatalysis applications. This WAR UC material showed a highly efficient UC process in the ambient environment owing to its liquid/solid encapsulation structure. In the application measurement, the UC emission from WAR UC materials can excite the catalyst Pt/WO3 to produce hydroxyl radicals, yielding 7-hydroxycoumarin by reacting with coumarin. In another case, since the band gap of ZnCdS matches the energy of UC emission, hole-electron pairs can be obtained under the UC irradiation and capture electrons from rhodamine B, leading to the degradation of rhodamine B. The maximum of the photocatalysis efficiency can be up to 97%. This work solves the oxygen quenching problem by preparing a triplet-triplet annihilation upconversion (TTA-UC) O/W microemulsion and loading it into PAAS WAR, and opens a new avenue to solid-state devices for TTA-UC. The applications of photocatalytic synthesis and photocatalytic degradation lay a foundation for future practical applications for TTA-UC materials.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Chen S. L. Stehr J. Reddy N. K. Tu C. W. Chen W. M. Buyanova I. A. Appl. Phys. B: Lasers Opt. 2012;108:919. doi: 10.1007/s00340-012-5138-y. - DOI
-
- Dong W. Zhou G. Xu X. Wang X. Liu Z. Yan R. Shao Z. Jiang M. Opt. Laser Technol. 2002;34:55. doi: 10.1016/S0030-3992(02)00092-0. - DOI
-
- Hasan Z. Biyikli L. Sellars M. J. Khodaparast G. A. Richardson F. S. Quagliano J. R. Phys. Rev. B: Condens. Matter Mater. Phys. 1997;56:4518. doi: 10.1103/PhysRevB.56.4518. - DOI
-
- Bao W. Sun B. Wang X. Ye C. Ping D. Liang Z. Chen Z. Tao X. Wu L. J. Phys. Chem. C. 2014;118:1417. doi: 10.1021/jp410984c. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

