Ruthenium-catalyzed, site-selective C-H activation: access to C5-substituted azaflavanone
- PMID: 35520642
- PMCID: PMC9056431
- DOI: 10.1039/d0ra06580c
Ruthenium-catalyzed, site-selective C-H activation: access to C5-substituted azaflavanone
Abstract
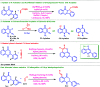
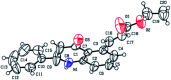
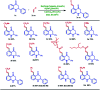
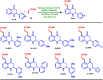
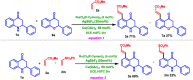
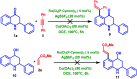
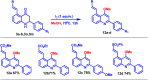
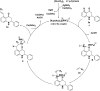
A site-selective ruthenium-catalyzed keto group assisted C-H bond activation of 2-aryl tetrahydroquinoline (azaflavanone) derivatives has been achieved with a variety of alkenes for the first time. A wide range of substrates was utilized for the synthesis of a wide variety of alkenylated azaflavanones. This simple and efficient protocol provides the C5-substituted azaflavanone derivatives in high yields with a broad range of functional group tolerance. Further, the C5-alkenylated products were converted into substituted 2-aryl quinoline derivatives in good yields.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Ru(II)-Catalyzed [4 + 2]-Annulation of 2-Alkenyl/Arylimidazoles with N-Substituted Maleimides and 1,4-Naphthoquinones: Access to Imidazo-Fused Polyheterocycles.J Org Chem. 2024 Feb 16;89(4):2272-2282. doi: 10.1021/acs.joc.3c02229. Epub 2024 Feb 2. J Org Chem. 2024. PMID: 38305185
-
Ruthenium-catalyzed remote C5-sulfonation of N-alkyl-8-aminoquinolines.Org Biomol Chem. 2019 Aug 28;17(32):7564-7568. doi: 10.1039/c9ob01357a. Epub 2019 Aug 2. Org Biomol Chem. 2019. PMID: 31373593
-
Synthesis of o-Alkenylated 2-Arylbenzoxazoles via Rh-Catalyzed Oxidative Olefination of 2-Arylbenzoxazoles: Scope Investigation, Structural Features, and Mechanism Studies.J Org Chem. 2016 Dec 16;81(24):12169-12180. doi: 10.1021/acs.joc.6b01836. Epub 2016 Nov 23. J Org Chem. 2016. PMID: 27978727
-
Recent advances in the ruthenium-catalyzed hydroarylation of alkynes with aromatics: synthesis of trisubstituted alkenes.Org Biomol Chem. 2015 Nov 14;13(42):10420-36. doi: 10.1039/c5ob01472g. Epub 2015 Sep 18. Org Biomol Chem. 2015. PMID: 26383714 Review.
-
Ruthenium-catalyzed direct arylations through C-H bond cleavages.Top Curr Chem. 2010;292:211-29. doi: 10.1007/128_2009_9. Top Curr Chem. 2010. PMID: 21500408 Review.
Cited by
-
Mono and di ortho-C-H acetoxylation of 2-aryloxyquinoline-3-carbaldehydes.RSC Adv. 2024 Apr 23;14(19):13306-13310. doi: 10.1039/d4ra01289e. eCollection 2024 Apr 22. RSC Adv. 2024. PMID: 38655482 Free PMC article.
-
Inherent Weakly Coordinating Oxo-Group-Directed Ruthenium(II)-Catalyzed C5 Functionalization of 2-Arylquinolin-4(1H)-ones.Org Lett. 2025 Aug 15;27(32):8881-8886. doi: 10.1021/acs.orglett.5c02418. Epub 2025 Aug 5. Org Lett. 2025. PMID: 40762573 Free PMC article.
-
Distal meta-C-H functionalization of α-substituted cinnamates.Chem Sci. 2023 Feb 23;14(22):5880-5886. doi: 10.1039/d2sc06206b. eCollection 2023 Jun 7. Chem Sci. 2023. PMID: 37293646 Free PMC article.
-
Recent advances in synthetic approaches for bioactive cinnamic acid derivatives.Beilstein J Org Chem. 2025 May 28;21:1031-1086. doi: 10.3762/bjoc.21.85. eCollection 2025. Beilstein J Org Chem. 2025. PMID: 40463886 Free PMC article. Review.
References
-
-
Selected review;
- Ma W. Gandeepan P. Li J. Ackermann L. Org. Chem. Front. 2017;4:1435. doi: 10.1039/C7QO00134G. - DOI
- Davies D. L. Macgregor S. A. McMullin C. L. Chem. Rev. 2017;117:8649. doi: 10.1021/acs.chemrev.6b00839. - DOI - PubMed
- Mishra N. K. Sharma S. Park J. Han S. Kim I. S. ACS Catal. 2017;7:2821. doi: 10.1021/acscatal.7b00159. - DOI
- Roudesly F. Oble J. Poli G. J. Mol. Catal. A: Chem. 2017;426:275. doi: 10.1016/j.molcata.2016.06.020. - DOI
- Yang Y. Lan J. You J. Chem. Rev. 2017;117:8787. doi: 10.1021/acs.chemrev.6b00567. - DOI - PubMed
- Xue X. S. Zhou P. Ji B. Cheng J. P. Chem. Rev. 2017;117:8622. doi: 10.1021/acs.chemrev.6b00664. - DOI - PubMed
- Dong Z. Ren Z. Thompson S. J. Xu Y. Dong G. Chem. Rev. 2017;117:9333. doi: 10.1021/acs.chemrev.6b00574. - DOI - PubMed
- Agasti S. Mondal B. Achar T. K. Sinha S. K. Suseelan A. S. Szabo K. J. Schoenebeck F. Maiti D. ACS Catal. 2019;9:9606. doi: 10.1021/acscatal.9b03019. - DOI
- Deb A. Bag S. Kancherla R. Maiti D. J. Am. Chem. Soc. 2014;136:13602. doi: 10.1021/ja5082734. - DOI - PubMed
-
-
-
Selected review;
- Arockiam P. Bruneau C. Dixneuf P. Chem. Rev. 2012;112:5879. doi: 10.1021/cr300153j. - DOI - PubMed
- Iwai T. Sawamura M. ACS Catal. 2015;5(9):5031. doi: 10.1021/acscatal.5b01143. - DOI
- Gensch T. Hopkinson M. N. Glorius F. Delord W. Chem. Soc. Rev. 2016;45:2900. doi: 10.1039/C6CS00075D. - DOI - PubMed
- Kakiuchi F. Tanaka Y. Sato T. Chatani N. Murai S. Chem. Lett. 1995:679. doi: 10.1246/cl.1995.679. - DOI
- Zhang M. Zhang Y. Jie X. Zhao H. Li G. Su W. Org. Chem. Front. 2014;1:843. doi: 10.1039/C4QO00068D. - DOI
- Lyons T. W. Sanford M. Chem. Rev. 2010;110:1147. doi: 10.1021/cr900184e. - DOI - PMC - PubMed
- Rousseau G. Breit B. Angew. Chem., Int. Ed. 2011;50:2450. doi: 10.1002/anie.201006139. - DOI - PubMed
- Bag S. Maiti D. Synthesis. 2016;48:804–815. doi: 10.1055/s-0035-1561321. - DOI
-
-
- Basavaiah D. Reddy B. S. Badsara S. S. Chem. Rev. 2010;110:5447. doi: 10.1021/cr900291g. - DOI - PubMed
- Bakthadoss M. Srinivasan J. Selvakumar R. Synthesis. 2012;44:755. doi: 10.1055/s-0031-1289707. - DOI
- Rotstein B. H. Zaretsky S. Rai V. Yudin A. K. Chem. Rev. 2014;114:8323. doi: 10.1021/cr400615v. - DOI - PubMed
- Basavaiah D. Pandiaraju S. Bakthadoss M. Muthukumaran K. Tetrahedron: Asymmetry. 1996;7:997. doi: 10.1016/0957-4166(96)00101-2. - DOI
- Bakthadoss M. Sivakumar G. Sharada D. S. Synthesis. 2013:237–245.
- Bakthadoss M. Sivakumar N. Devaraj A. Tetrahedron Lett. 2015;56:4980. doi: 10.1016/j.tetlet.2015.07.003. - DOI
- Marcos A. P. Clarissa P. Dayse N. Buriol M. MachadoKatritzky P. Chem. Rev. 2009;109:4140. doi: 10.1021/cr9001098. - DOI - PubMed
- Bakthadoss M. Devaraj A. Tetrahedron Lett. 2015;56:3954. doi: 10.1016/j.tetlet.2015.05.004. - DOI
- Bakthadoss M. Kannan D. Selvakumar R. Chem. Commun. 2013;49:10947. doi: 10.1039/C3CC45502E. - DOI - PubMed
- Wu P. Feldman A. K. Nugent A. K. et al. . Angew. Chem., Int. Ed. Engl. 2004;43:3928. doi: 10.1002/anie.200454078. - DOI - PubMed
- Basavaiah D. Bakthadoss M. Jayapal Reddy G. Synth. Commun. 2002;32:689. doi: 10.1081/SCC-120002506. - DOI
- Bakthadoss M. Sivakumar N. Devaraj A. Synthesis. 2011;4:0611. doi: 10.1055/s-0030-1258409. - DOI
- Li H. H. Liu C. Zhang Y. Sun Y. Wang B. Liu W. Org. Lett. 2015;17:932. doi: 10.1021/acs.orglett.5b00033. - DOI - PubMed
- Trost B. M. Tracy J. S. Org. Lett. 2017;19:2630. doi: 10.1021/acs.orglett.7b00961. - DOI - PMC - PubMed
- Bakthadoss M. Kannan D. Sivakumar N. Malathi P. Manikandan V. Org. Biomol. Chem. 2015;13:5597. doi: 10.1039/C5OB00442J. - DOI - PubMed
- Bakthadoss M. Selvakumar R. J. Org. Chem. 2016;81:3391. doi: 10.1021/acs.joc.5b02920. - DOI - PubMed
-
- Cole A. G. Metzger A. Brescia M. R. Qin L. Y. Appell K. C. Brain C. T. Hallett A. Ganju P. Denholm A. A. Wareing J. R. et al. . Bioorg. Med. Chem. Lett. 2009;19:119. doi: 10.1016/j.bmcl.2008.11.005. - DOI - PubMed
- Su D. S. Lim J. J. Tinney E. Wan B. L. Young M. B. Anderson K. D. Rudd D. Munshi V. Bahnck C. Felock P. J. et al. . Bioorg. Med. Chem. Lett. 2009;19:5119. doi: 10.1016/j.bmcl.2009.07.031. - DOI - PubMed
-
- Nammalwar B. Bunce R. A. Molecules. 2014;19:204. doi: 10.3390/molecules19010204. - DOI - PMC - PubMed
- Katritzky A. R. Rachwal S. Rachwal B. Tetrahedron. 1996;52:15031. doi: 10.1016/S0040-4020(96)00911-8. - DOI
- Sridharan V. Suryavanshi P. Menéndez J. C. Chem. Rev. 2011;111:7157. doi: 10.1021/cr100307m. - DOI - PubMed
- Muthukrishnan M. Sridharan V. Menéndez J. C. Chem. Rev. 2019;119(8):5057. doi: 10.1021/acs.chemrev.8b00567. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

