Development and characterization of a novel l-asparaginase/MWCNT nanobioconjugate
- PMID: 35520670
- PMCID: PMC9056397
- DOI: 10.1039/d0ra05534d
Development and characterization of a novel l-asparaginase/MWCNT nanobioconjugate
Abstract
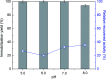
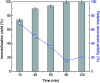
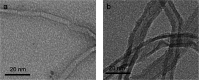
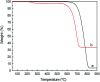
The enzyme l-asparaginase (ASNase) presents effective antineoplastic properties used for acute lymphoblastic leukemia treatment besides their potential use in the food sector to decrease the acrylamide formation. Considering their applications, the improvement of this enzyme's properties by efficient immobilization techniques is in high demand. Carbon nanotubes are promising enzyme immobilization supports, since these materials have increased surface area and effective capacity for enzyme loading. Accordingly, in this study, multi-walled carbon nanotubes (MWCNTs) were explored as novel supports for ASNase immobilization by a simple adsorption method. The effect of pH and contact time of immobilization, as well as the ASNase to nanoparticles mass ratio, were optimized according to the enzyme immobilization yield and relative recovered activity. The enzyme-MWCNTs bioconjugation was confirmed by thermogravimetric analysis (TGA), Fourier transform infrared spectroscopy (FTIR), Raman and transmission electron microscopy (TEM) studies. MWCNTs have a high ASNase loading capacity, with a maximum immobilization yield of 90%. The adsorbed ASNase retains 90% of the initial enzyme activity at the optimized conditions (pH 8.0, 60 min, and 1.5 × 10-3 g mL-1 of ASNase). According to these results, ASNase immobilized onto MWCNTs can find improved applications in several areas, namely biosensors, medicine and food industry.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
-
- Shakambari G. Sumi B. M. Ashokkumar B. Palanivelu P. Varalakshmi P. RSC Adv. 2015;5:48729–48738. doi: 10.1039/C5RA05507E. - DOI
-
- Shanmugaprakash M. Jayashree C. Vinothkumar V. Senthilkumar S. N. S. Siddiqui S. Rawat V. Arshad M. Sep. Purif. Technol. 2015;142:258–267. doi: 10.1016/j.seppur.2014.12.036. - DOI
-
- Shakambari G. Birendranarayan A. K. Angelaa Lincy M. J. Rai S. K. Ahamed Q. T. Ashokkumar B. Saravanan M. Mahesh A. Varalakshmi P. RSC Adv. 2016;6:25943–25951. doi: 10.1039/C6RA00727A. - DOI
LinkOut - more resources
Full Text Sources

