Synthesis, characterization and chemical degradation of poly(ester-triazole)s derived from d-galactose
- PMID: 35520726
- PMCID: PMC9062189
- DOI: 10.1039/c9ra00398c
Synthesis, characterization and chemical degradation of poly(ester-triazole)s derived from d-galactose
Abstract
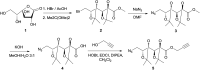
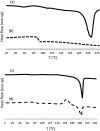
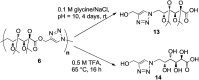
α-Azide-ω-alkynyl ester monomers were designed and synthesized in order to obtain hydrolytically degradable polymers. The monomers were prepared from d-galactose, as a renewable resource. Environmentally benign azido-alkyne cycloaddition polymerizations were conducted to afford poly(ester-triazole)s, with complete atom economy. Although polymer formation prevailed under optimized polymerization conditions, variable proportions of cyclic oligomer byproducts were detected. The Cu-catalyzed click polymerization led regioselectively to 1,4-disubstituted triazole linkages, while the thermal, metal-free polymerization produced a random distribution of 1,4- and 1,5-disubstituted triazoles in the polymer backbone. The poly(ester-triazole)s exhibited high molecular weights (M w in the range 35-85 kDa). They were soluble in organic solvents but highly insoluble in water, thus removal of the Cu(i) catalyst was simplified. The polymers were stable up to 300 °C, and had T g values in the range 90-100 °C. The materials were hydrolysed under either basic or strong acid conditions, and the degradation products have been characterized.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
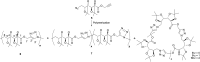
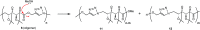


References
-
- Rostovtsev V. V. Green L. G. Fokin V. V. Sharpless K. B. Angew. Chem., Int. Ed. 2002;41:2596. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. - DOI - PubMed
- Tornøe C. W. Christensen C. Meldal M. J. Org. Chem. 2002;67:3057. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. - DOI - PubMed
-
- Meldal M. Macromol. Rapid Commun. 2008;29:1016. doi: 10.1002/marc.200800159. - DOI
-
- Huisgen R. Szeimies G. Möbius L. Chem. Ber. 1967;100:2494. doi: 10.1002/cber.19671000806. - DOI
LinkOut - more resources
Full Text Sources

