Selected pharmaceuticals removal using algae derived porous carbon: experimental, modeling and DFT theoretical insights
- PMID: 35520732
- PMCID: PMC9062196
- DOI: 10.1039/c9ra01086f
Selected pharmaceuticals removal using algae derived porous carbon: experimental, modeling and DFT theoretical insights
Abstract
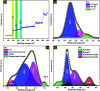
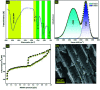
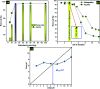
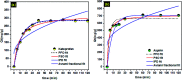
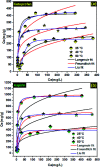
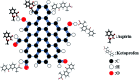
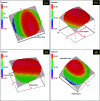
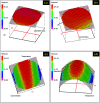
Porous carbon from Laminaria digitata algae activated using NaOH (PCLD@NaOH) was prepared by a chemical activation approach and has been tested for the adsorption of ketoprofen and aspirin molecules. The prepared PCLD@NaOH was characterized using XPS, FTIR, Raman, N2-physisorption, SEM, acidic/basic character (Boehm), and pHPZC. The batch adsorption of ketoprofen and aspirin was investigated under different parameters. The adsorption kinetics on PCLD@NaOH were well described by the Avrami-fractional kinetic model and the equilibrium data by Liu isotherm model. The adsorption capacity of aspirin (970.88 mg g-1 at 25 °C) was higher than ketoprofen (443.45 mg g-1 at 25 °C). The thermodynamic values indicate that the adsorption of ketoprofen and aspirin is exothermic and spontaneous. These results were in good agreement with DFT calculation that shows that the aspirin molecule presents high reactivity, electrophilicity, and softness compared to the ketoprofen molecule. Finally, the response surface methodology was used to optimize the removal efficiency of ketoprofen and aspirin.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
Authors declare no conflict of interest.
Figures




Similar articles
-
Characterization of activated carbon produced from the green algae Spirogyra used as a cost-effective adsorbent for enhanced removal of copper(ii): application in industrial wastewater treatment.RSC Adv. 2024 Feb 9;14(8):5276-5289. doi: 10.1039/d3ra08678j. eCollection 2024 Feb 7. RSC Adv. 2024. PMID: 38343995 Free PMC article.
-
Adsorption modeling, thermodynamics, and DFT simulation of tetracycline onto mesoporous and high-surface-area NaOH-activated macroalgae carbon.J Hazard Mater. 2022 Mar 5;425:127887. doi: 10.1016/j.jhazmat.2021.127887. Epub 2021 Nov 29. J Hazard Mater. 2022. PMID: 34906868
-
Development of activated carbon from Schizolobium parahyba (guapuruvu) residues employed for the removal of ketoprofen.Environ Sci Pollut Res Int. 2022 Mar;29(15):21860-21875. doi: 10.1007/s11356-021-17422-5. Epub 2021 Nov 12. Environ Sci Pollut Res Int. 2022. PMID: 34773238
-
Phenol adsorption on high microporous activated carbons prepared from oily sludge: equilibrium, kinetic and thermodynamic studies.Sci Rep. 2019 Dec 18;9(1):19352. doi: 10.1038/s41598-019-55794-4. Sci Rep. 2019. PMID: 31852930 Free PMC article.
-
Adsorption of caffeine on mesoporous activated carbon fibers prepared from pineapple plant leaves.Ecotoxicol Environ Saf. 2018 Jan;147:64-71. doi: 10.1016/j.ecoenv.2017.08.034. Epub 2017 Sep 14. Ecotoxicol Environ Saf. 2018. PMID: 28837871
Cited by
-
Ketoprofen as an emerging contaminant: occurrence, ecotoxicity and (bio)removal.Front Microbiol. 2023 Aug 7;14:1200108. doi: 10.3389/fmicb.2023.1200108. eCollection 2023. Front Microbiol. 2023. PMID: 37608946 Free PMC article. Review.
-
ZIF-8 metal organic framework materials as a superb platform for the removal and photocatalytic degradation of organic pollutants: a review.RSC Adv. 2022 Nov 7;12(49):31801-31817. doi: 10.1039/d2ra05717d. eCollection 2022 Nov 3. RSC Adv. 2022. PMID: 36380941 Free PMC article. Review.
-
Characterization of activated carbon produced from the green algae Spirogyra used as a cost-effective adsorbent for enhanced removal of copper(ii): application in industrial wastewater treatment.RSC Adv. 2024 Feb 9;14(8):5276-5289. doi: 10.1039/d3ra08678j. eCollection 2024 Feb 7. RSC Adv. 2024. PMID: 38343995 Free PMC article.
-
Microalgae-based bioremediation of refractory pollutants: an approach towards environmental sustainability.Microb Cell Fact. 2025 Jan 14;24(1):19. doi: 10.1186/s12934-024-02638-0. Microb Cell Fact. 2025. PMID: 39810167 Free PMC article. Review.
References
-
- Caroli S. Caracciolo A. B. Toxicol. Environ. Chem. 2010;92:549–565. doi: 10.1080/14786430903248912. - DOI
LinkOut - more resources
Full Text Sources

