Protection of silver and gold LSPR biosensors in corrosive NaCl environment by short alkanethiol molecules; characterized by extinction spectrum, helium ion microscopy and SERS
- PMID: 35520752
- PMCID: PMC9062164
- DOI: 10.1039/c8ra09778j
Protection of silver and gold LSPR biosensors in corrosive NaCl environment by short alkanethiol molecules; characterized by extinction spectrum, helium ion microscopy and SERS
Abstract
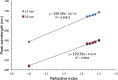
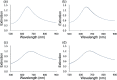
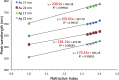
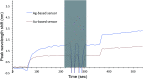
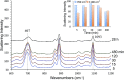
We investigated the utility of localized surface plasmon resonance sensors in a biologically relevant environment containing NaCl. Our sensors are fabricated by depositing gold or silver on a monolayer of adsorbed monodisperse SiO2 nanospheres. While silver nanostructures are rather unstable in air and water as assessed by drifts in the extinction peak, even gold nanostructures have been found to drift at elevated NaCl concentrations. In an attempt to protect these nanostructures against NaCl, we modified them with alkanethiols with different lengths in the vapor phase and found that shorter chain alkanethiols such as 1-butanethiol are particularly effective against even 250 mM NaCl, rather than longer-chain alkanethiols more suitable for robust SAM formation. A vapor phase treatment method, in contrast to widely used solution phase treatment methods, was selected with the intention of reducing the solvent effect, i.e. destruction of intricate nanostructures upon contact with a solvent when nanostructures have been prepared in a vacuum system. Moreover, the treatment with 1-butanethiol led to an enhanced sensitivity as expressed by peak shift in nm per refractive index unit, nm per RIU. We show the results of evaluating alkanethiol-protected silver and gold nanostructures by extinction spectroscopy, helium ion microscopy and surface-enhanced Raman spectroscopy. The vapor phase treatment method with short chain alkanethiols is an effective way to protect intricate gold and silver nanostructures prepared in a vacuum system.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

