Rapid and halide compatible synthesis of 2- N-substituted indazolone derivatives via photochemical cyclization in aqueous media
- PMID: 35520758
- PMCID: PMC9063774
- DOI: 10.1039/c9ra02466b
Rapid and halide compatible synthesis of 2- N-substituted indazolone derivatives via photochemical cyclization in aqueous media
Abstract
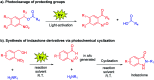
Indazolone derivatives exhibit a wide range of biological and pharmaceutical properties. We report a rapid and efficient approach to provide structurally diverse 2-N-substituted indazolones via photochemical cyclization in aqueous media at room temperature. This straightforward protocol is halide compatible for the synthesis of halogenated indazolones bearing a broad scope of substrates, which suggests a new avenue of great importance to medicinal chemistry.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


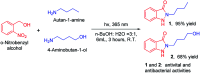
Similar articles
-
A B2(OH)4-Mediated Synthesis of 2-Substituted Indazolone and Its Application in a DNA-Encoded Library.Org Lett. 2020 Aug 21;22(16):6277-6282. doi: 10.1021/acs.orglett.0c02032. Epub 2020 Aug 1. Org Lett. 2020. PMID: 32806212
-
IBX-mediated synthesis of indazolone via oxidative N-N bond formation and unexpected formation of quinazolin-4-one: in situ generation of formaldehyde from dimethoxyethane.Arch Pharm Res. 2016 Mar;39(3):302-9. doi: 10.1007/s12272-016-0706-z. Epub 2016 Jan 16. Arch Pharm Res. 2016. PMID: 26780246
-
N-N Bond Formation between Primary Amines and Nitrosos: Direct Synthesis of 2-Substituted Indazolones with Mechanistic Insights.Org Lett. 2018 Aug 17;20(16):4736-4739. doi: 10.1021/acs.orglett.8b01655. Epub 2018 Aug 1. Org Lett. 2018. PMID: 30067041 Free PMC article.
-
Davis-Beirut Reaction: Diverse Chemistries of Highly Reactive Nitroso Intermediates in Heterocycle Synthesis.Acc Chem Res. 2019 Aug 20;52(8):2256-2265. doi: 10.1021/acs.accounts.9b00220. Epub 2019 Jul 22. Acc Chem Res. 2019. PMID: 31328502 Free PMC article. Review.
-
Indole synthesis by radical cyclization of o-alkenylphenyl isocyanides and its application to the total synthesis of natural products.Chem Rec. 2002;2(1):37-45. doi: 10.1002/tcr.10008. Chem Rec. 2002. PMID: 11933260 Review.
Cited by
-
A photochemical C=C cleavage process: toward access to backbone N-formyl peptides.Beilstein J Org Chem. 2021 Dec 15;17:2932-2938. doi: 10.3762/bjoc.17.202. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 34956413 Free PMC article.
-
General Platform for Efficient and Modular Assembly of GalNAc-siRNA Conjugates via Primary Amines and o-Nitrobenzyl Alcohol Cyclization Photoclick Chemistry Enabling Rapid Access to Therapeutic Oligonucleotides.JACS Au. 2025 Feb 24;5(3):1402-1412. doi: 10.1021/jacsau.5c00012. eCollection 2025 Mar 24. JACS Au. 2025. PMID: 40151235 Free PMC article.
-
Continuous Flow Synthesis of Nitrosoarenes via Photochemical Rearrangement of Aryl Imines.J Org Chem. 2024 Jan 5;89(1):617-623. doi: 10.1021/acs.joc.3c02362. Epub 2023 Dec 22. J Org Chem. 2024. PMID: 38131303 Free PMC article.
-
Electrochemical synthesis of N,N'-disubstituted indazolin-3-ones via an intramolecular anodic dehydrogenative N-N coupling reaction.Chem Sci. 2022 Jun 13;13(27):8180-8186. doi: 10.1039/d2sc01827f. eCollection 2022 Jul 13. Chem Sci. 2022. PMID: 35919432 Free PMC article.
-
Synthesis and Anti-Inflammatory Activity of N(2)-Arylindazol-3(2H)-One Derivatives: Copper-Promoted Direct N-Arylation via Chan-Evans-Lam Coupling.Molecules. 2023 Sep 20;28(18):6706. doi: 10.3390/molecules28186706. Molecules. 2023. PMID: 37764482 Free PMC article.
References
-
- Zhang S. G. Liang C. G. Zhang W. H. Molecules. 2018;23:2783. doi: 10.3390/molecules23112783. - DOI - PMC - PubMed
- Denya I. Malan S. F. Joubert J. Expert Opin. Ther. Pat. 2018;28:441. doi: 10.1080/13543776.2018.1472240. - DOI - PubMed
- Thangadurai A. Minu M. Wakode S. Agrawal S. Narasimhan B. Med. Chem. Res. 2012;21:1509–1523. doi: 10.1007/s00044-011-9631-3. - DOI
- Haddadin M. J. Conrad W. E. Kurth M. J. Mini-Rev. Med. Chem. 2012;12:1293. doi: 10.2174/138955712802762059. - DOI - PubMed
- Dong J. Y. Zhang Q. J. Wang Z. T. Huang G. Li S. S. ChemMedChem. 2018;13:1490. doi: 10.1002/cmdc.201800253. - DOI - PubMed
- Gaikwad D. D. Chapolikar A. D. Devkate C. G. Warad K. D. Tayade A. P. Pawar R. P. Domb A. J. Eur. J. Med. Chem. 2015;90:707. doi: 10.1016/j.ejmech.2014.11.029. - DOI - PubMed
-
- Montero-Torres A. Vega M. C. Marrero-Ponce Y. Rolon M. Gomez-Barrio A. Escario J. A. Aran V. J. Martinez-Fernandez A. R. Meneses-Marcel A. Bioorg. Med. Chem. 2005;13:6264. doi: 10.1016/j.bmc.2005.06.049. - DOI - PubMed
- Vega M. C. Rolon M. Montero-Torres A. Fonseca-Berzal C. Escario J. A. Gomez-Barrio A. Galvez J. Marrero-Ponce Y. Aran V. J. Eur. J. Med. Chem. 2012;58:214. doi: 10.1016/j.ejmech.2012.10.009. - DOI - PubMed
LinkOut - more resources
Full Text Sources

