Immobilized antimony species on magnetite: a novel and highly efficient magnetically reusable nanocatalyst for direct and gram-scale reductive-coupling of nitroarenes to azoarenes
- PMID: 35520760
- PMCID: PMC9063975
- DOI: 10.1039/c9ra01249d
Immobilized antimony species on magnetite: a novel and highly efficient magnetically reusable nanocatalyst for direct and gram-scale reductive-coupling of nitroarenes to azoarenes
Abstract
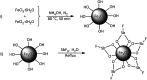
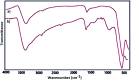
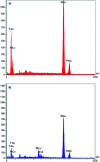
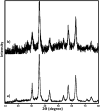
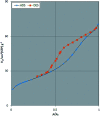
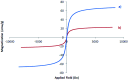
In this study, magnetic nanoparticles of Fe3O4@SbF x from the immobilization of SbF3 on magnetite were synthesized. The prepared nanocomposite system was then characterized using scanning electron microscopy, Fourier transform infrared spectroscopy, X-ray diffraction, energy-dispersive X-ray spectroscopy, vibrating sample magnetometry and inductively coupled plasma optical emission spectroscopy. Next, the catalytic activity of Fe3O4@SbF x MNPs was highlighted by one-pot reductive-coupling of aromatic nitro compounds to the corresponding azoarene materials with NaBH4. The reactions were carried out in refluxing EtOH within 6-25 min to afford the products in high yields. The reusability of the Sb-magnetite system was also studied for 6 consecutive cycles without significant loss of catalytic activity. This synthetic protocol provided several advantages in terms of introducing a novel catalytic system based on antimony species for direct and gram-scale preparation of azoarenes from nitroarenes, low loading of the nanocatalyst, mild reaction conditions, using ethanol as a green and economic solvent and high yield of the products.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
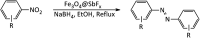

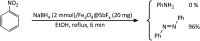
Similar articles
-
A uniformly anchored zirconocene complex on magnetic reduced graphene oxide (rGO@Fe3O4/ZrCp2Cl x (x = 0, 1, 2)) as a novel and reusable nanocatalyst for synthesis of N-arylacetamides and reductive-acetylation of nitroarenes.RSC Adv. 2022 May 17;12(24):15020-15037. doi: 10.1039/d2ra02293a. eCollection 2022 May 17. RSC Adv. 2022. PMID: 35702429 Free PMC article.
-
Sonochemical in situ immobilization of Pd nanoparticles on green tea extract coated Fe3O4 nanoparticles: An efficient and magnetically recyclable nanocatalyst for synthesis of biphenyl compounds under ultrasound irradiations.Mater Sci Eng C Mater Biol Appl. 2019 May;98:584-593. doi: 10.1016/j.msec.2019.01.009. Epub 2019 Jan 4. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 30813061
-
Quinoline-2-carboimine copper complex immobilized on amine functionalized silica coated magnetite nanoparticles: a novel and magnetically retrievable catalyst for the synthesis of carbamates via C-H activation of formamides.Dalton Trans. 2015 Jan 21;44(3):1303-16. doi: 10.1039/c4dt03236e. Dalton Trans. 2015. PMID: 25417959
-
In situ decoration of Au NPs over polydopamine encapsulated GO/Fe3O4 nanoparticles as a recyclable nanocatalyst for the reduction of nitroarenes.Sci Rep. 2021 Jun 11;11(1):12362. doi: 10.1038/s41598-021-90514-x. Sci Rep. 2021. PMID: 34117274 Free PMC article.
-
Diverse and efficient catalytic applications of new cockscomb flower-like Fe3O4@SiO2@KCC-1@MPTMS@CuII mesoporous nanocomposite in the environmentally benign reduction and reductive acetylation of nitroarenes and one-pot synthesis of some coumarin compounds.RSC Adv. 2022 Apr 20;12(18):11164-11189. doi: 10.1039/d1ra08763k. eCollection 2022 Apr 7. RSC Adv. 2022. PMID: 35479105 Free PMC article.
References
-
- Hunger K., Industrial Dyes: Chemistry, Properties, Applications, Wiley-VCH, Weinheim, 2003
-
- Zollinger H., Colour Chemistry: Syntheses, Properties and Applications of Organic Dyes and Pigments, Wiley-VCH, Switzerland, 3rd edn, 2003
-
- Gordon P. F. and Gregory P., Organic Chemistry in Colour, Springer, New York, 1983, pp. 95–162
-
- Athey Jr R. D. Eur. Coat. J. 1998;3:146.
-
- Ashutosh P. N. D. Mehrotra J. K. Colourage. 1979;26:25.
LinkOut - more resources
Full Text Sources

