Plasma functional polymerization of dopamine using atmospheric pressure plasma and a dopamine solution mist
- PMID: 35520781
- PMCID: PMC9063741
- DOI: 10.1039/c8ra10391g
Plasma functional polymerization of dopamine using atmospheric pressure plasma and a dopamine solution mist
Abstract
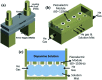
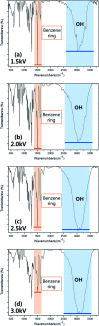
By using DBD-type atmospheric pressure plasmas and a dopamine solution mist formed by a piezoelectric module, the possibility of depositing functional polymer films showing the physical and chemical characteristics of polydopamine without breaking the functional group of the dopamine has been investigated for different plasma voltages. The higher DBD voltages up to 3.0 kV decreased the functional groups such as catechol and amine (N/C ratio) relative to dopamine in the deposited polymer by increasing the dissociation of dopamine into atoms and small molecules due to higher electron energies. In contrast, the lower DBD voltages up to 1.5 kV increased the functional group and N/C ratio of dopamine in the deposited polymer by keeping the molecular structures of the dopamine due to lower electron energies. Therefore, the polymer deposited at the lower DBD voltages showed lower contact angles and higher metal absorption properties which are some of the surface modification characteristics of polydopamine. When the metal absorption properties of the polydopamine-like film deposited using the atmospheric pressure plasma of a low DBD voltage with a dopamine solution mist were compared with other metal absorbers for Cu, As, and Cr, the polydopamine-like film exhibited superior metal absorption properties. It is believed that this atmospheric pressure plasma process can be also applied to the plasma polymerization of other monomers without breaking the functional groups of the monomers.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures










Similar articles
-
Free-standing polydopamine films generated in the presence of different metallic ions: the comparison of reaction process and film properties.RSC Adv. 2018 May 18;8(33):18347-18354. doi: 10.1039/c8ra02930j. eCollection 2018 May 17. RSC Adv. 2018. PMID: 35541137 Free PMC article.
-
Surface characteristics of a self-polymerized dopamine coating deposited on hydrophobic polymer films.Langmuir. 2011 Dec 6;27(23):14180-7. doi: 10.1021/la202877k. Epub 2011 Nov 8. Langmuir. 2011. PMID: 22011109
-
Kinetics of polydopamine film deposition as a function of pH and dopamine concentration: insights in the polydopamine deposition mechanism.J Colloid Interface Sci. 2012 Nov 15;386(1):366-72. doi: 10.1016/j.jcis.2012.07.030. Epub 2012 Jul 20. J Colloid Interface Sci. 2012. PMID: 22874639
-
From Basics to Frontiers: A Comprehensive Review of Plasma-Modified and Plasma-Synthesized Polymer Films.Polymers (Basel). 2023 Aug 30;15(17):3607. doi: 10.3390/polym15173607. Polymers (Basel). 2023. PMID: 37688233 Free PMC article. Review.
-
Bioinspired dopamine and zwitterionic polymers for non-fouling surface engineering.Chem Soc Rev. 2021 Oct 18;50(20):11668-11683. doi: 10.1039/d1cs00658d. Chem Soc Rev. 2021. PMID: 34477190 Review.
Cited by
-
Enhancing Selectivity with Molecularly Imprinted Polymers via Non-Thermal Dielectric Barrier Discharge Plasma.Polymers (Basel). 2024 Aug 22;16(16):2380. doi: 10.3390/polym16162380. Polymers (Basel). 2024. PMID: 39204601 Free PMC article.
-
Control of Intermolecular Interactions toward the Production of Free-Standing Interfacial Polydopamine Films.ACS Appl Mater Interfaces. 2023 Aug 2;15(30):36922-36935. doi: 10.1021/acsami.3c05236. Epub 2023 Jul 25. ACS Appl Mater Interfaces. 2023. PMID: 37489635 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

