Structural insights into the calcium dependence of Stig cyclases
- PMID: 35520811
- PMCID: PMC9063808
- DOI: 10.1039/c9ra00960d
Structural insights into the calcium dependence of Stig cyclases
Abstract
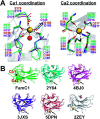
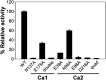
The Stig cyclases from Stigonematalean cyanobacteria are classified as a novel type of calcium-dependent cyclases which catalyze an uncommon reaction cascade comprising Cope rearrangement, 6-exo-trig cyclization, and electrophilic aromatic substitution. Previously we found two calcium ions near the substrate-binding pocket. The calcium-coordinating residues are conserved in all Stig cyclases. In the present study, we use site-directed mutagenesis to investigate the role of calcium coordination. By individually mutating the coordinating residues in either of the Ca2+-binding sites to alanine, the enzyme activity is significantly reduced, suggesting that the presence of Ca2+ in both sites is essential for catalysis. Furthermore, the crystal structure of N137A, in which the Ca2+-binding N137 is replaced by Ala, shows significant local conformational changes, resulting in a squeezed substrate-binding pocket that makes substrate entry ineffective. In conclusion, calcium coordination is important in setting up the structural elements for catalysis. These results add to the fundamental understanding of the mechanism of action of the calcium-dependent Stig cyclases.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors claim no conflict of interest.
Figures


Similar articles
-
Control of Stereoselectivity in Diverse Hapalindole Metabolites is Mediated by Cofactor-Induced Combinatorial Pairing of Stig Cyclases.Angew Chem Int Ed Engl. 2020 May 18;59(21):8166-8172. doi: 10.1002/anie.201913686. Epub 2020 Mar 19. Angew Chem Int Ed Engl. 2020. PMID: 32052896 Free PMC article.
-
The Crystal Structure of a Class of Cyclases that Catalyze the Cope Rearrangement.Angew Chem Int Ed Engl. 2018 Nov 12;57(46):15060-15064. doi: 10.1002/anie.201808231. Epub 2018 Oct 23. Angew Chem Int Ed Engl. 2018. PMID: 30222239
-
Structural basis of the Cope rearrangement and cyclization in hapalindole biogenesis.Nat Chem Biol. 2018 Apr;14(4):345-351. doi: 10.1038/s41589-018-0003-x. Epub 2018 Mar 12. Nat Chem Biol. 2018. PMID: 29531360 Free PMC article.
-
Terpenoid cyclases: design and function of electrophilic catalysts.Ciba Found Symp. 1992;171:163-76; discussion 176-83. doi: 10.1002/9780470514344.ch10. Ciba Found Symp. 1992. PMID: 1302176 Review.
-
Site-directed mutagenesis and substrate compatibility to reveal the structure-function relationships of plant oxidosqualene cyclases.Nat Prod Rep. 2021 Dec 15;38(12):2261-2275. doi: 10.1039/d1np00015b. Nat Prod Rep. 2021. PMID: 33988197 Review.
Cited by
-
Control of Stereoselectivity in Diverse Hapalindole Metabolites is Mediated by Cofactor-Induced Combinatorial Pairing of Stig Cyclases.Angew Chem Int Ed Engl. 2020 May 18;59(21):8166-8172. doi: 10.1002/anie.201913686. Epub 2020 Mar 19. Angew Chem Int Ed Engl. 2020. PMID: 32052896 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

