Two-photon absorption and two-photon-induced isomerization of azobenzene compounds
- PMID: 35520821
- PMCID: PMC9057575
- DOI: 10.1039/d0ra07693g
Two-photon absorption and two-photon-induced isomerization of azobenzene compounds
Abstract
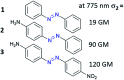
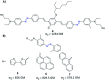
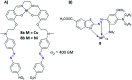
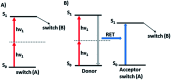
The process of two-photon-induced isomerization occurring in various organic molecules, among which azobenzene derivatives hold a prominent position, offers a wide range of functionalities, which can be used in both material and life sciences. This review provides a comprehensive description of nonlinear optical (NLO) properties of azobenzene (AB) derivatives whose geometries can be switched through two-photon absorption (TPA). Employing the nonlinear excitation process allows for deeper penetration of light into the tissues and provides opportunities to regulate biological systems in a non-invasive manner. At the same time, the tight focus of the beam needed to induce nonlinear absorption helps to improve the spatial resolution of the photoinduced structures. Since near-infrared (NIR) wavelengths are employed, the lower photon energies compared to usual one-photon excitation (typically, the azobenzene geometry change from trans to cis form requires the use of UV photons) cause less damage to the biological samples. Herein, we present an overview of the strategies for optimizing azobenzene-based photoswitches for efficient two-photon excitation (TPE) and the potential applications of two-photon-induced isomerization of azobenzenes in biological systems: control of ion flow in ion channels or control of drug release, as well as in materials science, to fabricate data storage media, optical filters, diffraction elements etc., based on phenomena like photoinduced anisotropy, mass transport and phase transition. The extant challenges in the field of two-photon switchable azomolecules are discussed.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures











Similar articles
-
Two-photon isomerization triggers two-photon-excited fluorescence of an azobenzene derivative.Spectrochim Acta A Mol Biomol Spectrosc. 2019 Jan 5;206:120-125. doi: 10.1016/j.saa.2018.07.098. Epub 2018 Aug 1. Spectrochim Acta A Mol Biomol Spectrosc. 2019. PMID: 30092493
-
Two-Photon-Induced versus One-Photon-Induced Isomerization Dynamics of a Bistable Azobenzene Derivative in Solution.J Phys Chem B. 2015 Sep 17;119(37):12281-8. doi: 10.1021/acs.jpcb.5b07008. Epub 2015 Sep 8. J Phys Chem B. 2015. PMID: 26322965
-
Rationally designed azobenzene photoswitches for efficient two-photon neuronal excitation.Nat Commun. 2019 Feb 22;10(1):907. doi: 10.1038/s41467-019-08796-9. Nat Commun. 2019. PMID: 30796228 Free PMC article.
-
Photoinduced Reversible Solid-to-Liquid Transitions for Photoswitchable Materials.Angew Chem Int Ed Engl. 2019 Jul 15;58(29):9712-9740. doi: 10.1002/anie.201814441. Epub 2019 Jun 18. Angew Chem Int Ed Engl. 2019. PMID: 30737869 Review.
-
Triggered azobenzene-based prodrugs and drug delivery systems.J Control Release. 2022 May;345:475-493. doi: 10.1016/j.jconrel.2022.03.041. Epub 2022 Mar 23. J Control Release. 2022. PMID: 35339578 Review.
Cited by
-
Spatially selective actuation of liquid-crystalline polymer films through two-photon absorption processes.Nat Commun. 2024 Nov 7;15(1):9430. doi: 10.1038/s41467-024-53682-8. Nat Commun. 2024. PMID: 39511191 Free PMC article.
-
Discovering Photoswitchable Molecules for Drug Delivery with Large Language Models and Chemist Instruction Training.Pharmaceuticals (Basel). 2024 Sep 30;17(10):1300. doi: 10.3390/ph17101300. Pharmaceuticals (Basel). 2024. PMID: 39458941 Free PMC article.
-
Detour to success: photoswitching via indirect excitation.Chem Sci. 2024 Jul 2;15(30):11684-11698. doi: 10.1039/d4sc02538e. eCollection 2024 Jul 31. Chem Sci. 2024. PMID: 39092110 Free PMC article. Review.
-
Chromophore Planarity, -BH Bridge Effect, and Two-Photon Activity: Bi- and Ter-Phenyl Derivatives as a Case Study.J Phys Chem A. 2023 Sep 28;127(38):7928-7936. doi: 10.1021/acs.jpca.3c04288. Epub 2023 Sep 18. J Phys Chem A. 2023. PMID: 37721870 Free PMC article.
-
Theoretical study of cis-trans isomer of 2-hydroxy-5-methyl-2'-nitroazobenzene: DFT insight.J Mol Model. 2023 May 15;29(6):177. doi: 10.1007/s00894-023-05583-8. J Mol Model. 2023. PMID: 37188843
References
-
- Lubbe A. S. van Leeuwen T. Wezenberg S. J. Feringa B. L. Tetrahedron. 2017;73:4837–4848. doi: 10.1016/j.tet.2017.06.049. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

