An organic-inorganic hybrid nanomaterial composed of a Dowson-type (NH4)6P2Mo18O62 heteropolyanion and a metal-organic framework: synthesis, characterization, and application as an effective adsorbent for the removal of organic dyes
- PMID: 35520823
- PMCID: PMC9057490
- DOI: 10.1039/d0ra07042d
An organic-inorganic hybrid nanomaterial composed of a Dowson-type (NH4)6P2Mo18O62 heteropolyanion and a metal-organic framework: synthesis, characterization, and application as an effective adsorbent for the removal of organic dyes
Abstract
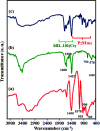
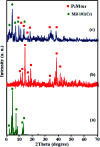
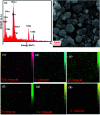
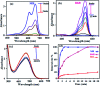
In this work, an inorganic-organic hybrid nanomaterial, P2Mo18/MIL-101(Cr), based on Wells-Dawson-type (NH4)6P2Mo18O62 polyoxometalate (abbreviated as P2Mo18) and the MIL-101(Cr) metal-organic framework was fabricated by the reaction of (NH4)6P2Mo18O62, Cr(NO3)3·9H2O and terephthalic acid under hydrothermal conditions. The as-prepared recyclable nanohybrid was fully characterized using X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FT-IR) equipped with energy dispersive X-ray microanalysis (EDX), field emission scanning electron microscopy (FE-SEM), Raman spectroscopy and Brunauer-Emmett-Teller (BET) specific surface area studies. All the analyses confirmed the successful insertion of P2Mo18O62 6- heteropolyanion within the cavities of MIL-101(Cr). The encapsulated MIL-101(Cr) showed a considerable decrease in both pore volume and surface area compared with MIL-101(Cr) due to incorporation of the very large Dowson-type polyoxometalate into the three-dimensional porous MIL-101(Cr). The nanohybrid had a specific surface area of 800.42 m2 g-1. The adsorption efficiency of this nanohybrid for removal of methylene blue (MB), rhodamine B (RhB), and methyl orange (MO) from aqueous solutions was evaluated. Surprisingly, the composite not only presented a high adsorption capacity of 312.5 mg g-1 for MB, but also has the ability to rapidly remove 100% MB from a dye solution of 50 mg L-1 within 3 min. These results confirmed that this adsorbent is applicable in a wide pH range of 2-10. The nanohybrid showed rapid and selective adsorption for cationic MB and RhB dyes from MB/MO, MB/RhB, MO/RhB and MB/MO/RhB mixed dye solutions. The equilibrium adsorption data were better fitted by the Langmuir isotherm. Kinetics data indicate that the adsorption of the dye follows a pseudo-second order kinetics model. Also, this material could be effortlessly separated and recycled without any structural modification. Accordingly, it is an efficient adsorbent for removing cationic dyes.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures









Similar articles
-
Dawson-Type Polyoxometalate Incorporated into Nanoporous MIL-101(Cr): Preparation, Characterization and Application for Ultrafast Removal of Organic Dyes.Acta Chim Slov. 2019 Feb;66(1):85-102. Acta Chim Slov. 2019. PMID: 33855468
-
K6P2W18O62 encapsulated into magnetic Fe3O4/MIL-101 (Cr) metal-organic framework: a novel magnetically recoverable nanoporous adsorbent for ultrafast treatment of aqueous organic pollutants solutions.RSC Adv. 2018 Nov 12;8(66):37976-37992. doi: 10.1039/c8ra06287k. eCollection 2018 Nov 7. RSC Adv. 2018. PMID: 35558601 Free PMC article.
-
Preparation and characterization of novel polyoxometalate/CoFe2O4/metal-organic framework magnetic core-shell nanocomposites for the rapid removal of organic dyes from water.RSC Adv. 2020 Nov 2;10(65):39881-39893. doi: 10.1039/d0ra04603e. eCollection 2020 Oct 27. RSC Adv. 2020. PMID: 35515376 Free PMC article.
-
Evolution of graphene oxide (GO)-based nanohybrid materials with diverse compositions: an overview.RSC Adv. 2022 Feb 16;12(9):5686-5719. doi: 10.1039/d1ra06731a. eCollection 2022 Feb 10. RSC Adv. 2022. PMID: 35425552 Free PMC article. Review.
-
Advances in Nanohybrid Membranes for Dye Reduction: A Comprehensive Review.Glob Chall. 2023 Dec 21;8(1):2300052. doi: 10.1002/gch2.202300052. eCollection 2024 Jan. Glob Chall. 2023. PMID: 38223886 Free PMC article. Review.
Cited by
-
Synthesis, characterization, and application of mixed-addenda silicon vanado tungstate polyoxometalate integrated into nanoporous MIL-101(Cr) for the quick removal of organic dyes from water.RSC Adv. 2025 Mar 24;15(12):8918-8930. doi: 10.1039/d5ra00443h. eCollection 2025 Mar 21. RSC Adv. 2025. PMID: 40129643 Free PMC article.
-
Construction of magnetic MoS2/NiFe2O4/MIL-101(Fe) hybrid nanostructures for separation of dyes and antibiotics from aqueous media.RSC Adv. 2024 Apr 5;14(16):11037-11056. doi: 10.1039/d4ra00505h. eCollection 2024 Apr 3. RSC Adv. 2024. PMID: 38586447 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

