An antibacterial and injectable calcium phosphate scaffold delivering human periodontal ligament stem cells for bone tissue engineering
- PMID: 35520873
- PMCID: PMC9057516
- DOI: 10.1039/d0ra06873j
An antibacterial and injectable calcium phosphate scaffold delivering human periodontal ligament stem cells for bone tissue engineering
Abstract
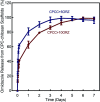
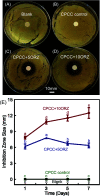
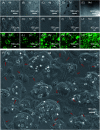
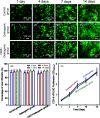
Osteomyelitis and post-operative infections are major problems in orthopedic, dental and craniofacial surgeries. It is highly desirable for a tissue engineering construct to kill bacteria, while simultaneously delivering stem cells and enhancing cell function and tissue regeneration. The objectives of this study were to: (1) develop a novel injectable calcium phosphate cement (CPC) scaffold containing antibiotic ornidazole (ORZ) while encapsulating human periodontal ligament stem cells (hPDLSCs), and (2) investigate the inhibition efficacy against Staphylococcus aureus (S. aureus) and the promotion of hPDLSC function for osteogenesis for the first time. ORZ was incorporated into a CPC-chitosan scaffold. hPDLSCs were encapsulated in alginate microbeads (denoted hPDLSCbeads). The ORZ-loaded CPCC+hPDLSCbeads scaffold was fully injectable, and had a flexural strength of 3.50 ± 0.92 MPa and an elastic modulus of 1.30 ± 0.45 GPa, matching those of natural cancellous bone. With 6 days of sustained ORZ release, the CPCC+10ORZ (10% ORZ) scaffold had strong antibacterial effects on S. aureus, with an inhibition zone of 12.47 ± 1.01 mm. No colonies were observed in the CPCC+10ORZ group from 3 to 7 days. ORZ-containing scaffolds were biocompatible with hPDLSCs. CPCC+10ORZ+hPDLSCbeads scaffold with osteogenic medium had 2.4-fold increase in alkaline phosphatase (ALP) activity and bone mineral synthesis by hPDLSCs, as compared to the control group (p < 0.05). In conclusion, the novel antibacterial construct with stem cell delivery had injectability, good strength, strong antibacterial effects and biocompatibility, supporting osteogenic differentiation and bone mineral synthesis of hPDLSCs. The injectable and mechanically-strong CPCC+10ORZ+hPDLSCbeads construct has great potential for treating bone infections and promoting bone regeneration.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





References
-
- Jayasree R. Kumar T. S. Perumal G. Doble M. Ceram. Int. 2018;44:9227–9235. doi: 10.1016/j.ceramint.2018.02.133. - DOI
LinkOut - more resources
Full Text Sources

