Synthesis and biological activities of petrosiols B and D
- PMID: 35520890
- PMCID: PMC9062523
- DOI: 10.1039/c9ra01166h
Synthesis and biological activities of petrosiols B and D
Abstract
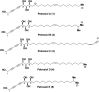
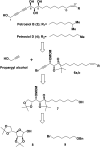
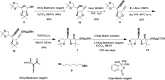
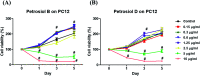
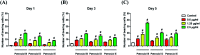
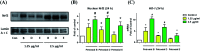
A divergent total synthesis of natural diacetylenic tetraols, petrosiol B and petrosiol D, was accomplished by taking advantage of a carbohydrate chiral template. In particular, petrosiol B, which is the first total synthesis so far, was achieved in 13 linear steps with a 10% overall yield applying Ohira-Bestmann homologation, NaH-mediated dehydrobromination, and Cu(i)-catalyzed Cadiot-Chodkiewicz coupling as the key reaction steps. The synthetic petrosiols B and D were subjected to the study on differentiation activities toward neuronal progenitor PC12 cells. Our results suggested that both petrosiol B and petrosiol D could induce the differentiation of neuronal progenitor PC12 cells via the enhancement of Nrf2 activity. By comparing petrosiols B, D and their natural homologue E, petrosiol B displayed the most intensive cell differentiation activity and the highest Nrf2 activity enhancement as well.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
An Important Function of Petrosiol E in Inducing the Differentiation of Neuronal Progenitors and in Protecting Them against Oxidative Stress.Adv Sci (Weinh). 2017 Jul 5;4(10):1700089. doi: 10.1002/advs.201700089. eCollection 2017 Oct. Adv Sci (Weinh). 2017. PMID: 29051854 Free PMC article.
-
Stereoselective total synthesis and structural revision of the diacetylenic diol natural products strongylodiols H and I.Beilstein J Org Chem. 2018 Sep 4;14:2313-2320. doi: 10.3762/bjoc.14.206. eCollection 2018. Beilstein J Org Chem. 2018. PMID: 30254695 Free PMC article.
-
Recent trends and applications of the Cadiot-Chodkiewicz reaction.Org Biomol Chem. 2019 Oct 23;17(41):9081-9094. doi: 10.1039/c9ob01757g. Org Biomol Chem. 2019. PMID: 31596306 Review.
-
Sponge-derived acetylenic alcohols, petrosiols, inhibit proliferation and migration of platelet-derived growth factor (PDGF)-induced vascular smooth muscle cells.Bioorg Med Chem. 2013 Apr 1;21(7):1804-10. doi: 10.1016/j.bmc.2013.01.039. Epub 2013 Jan 31. Bioorg Med Chem. 2013. PMID: 23415061
-
The Bestmann-Ohira Reagent and Related Diazo Compounds for the Synthesis of Azaheterocycles.Chem Rec. 2020 Nov;20(11):1394-1408. doi: 10.1002/tcr.202000091. Epub 2020 Sep 28. Chem Rec. 2020. PMID: 32986304 Review.
Cited by
-
Neurotrophic Natural Products.Prog Chem Org Nat Prod. 2024;123:1-473. doi: 10.1007/978-3-031-42422-9_1. Prog Chem Org Nat Prod. 2024. PMID: 38340248
-
Corey-Fuchs reaction enabled synthesis of natural products: a review.RSC Adv. 2025 Mar 17;15(11):8121-8155. doi: 10.1039/d5ra00619h. eCollection 2025 Mar 17. RSC Adv. 2025. PMID: 40103970 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

