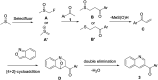
An efficient 3-acylquinoline synthesis from acetophenones and anthranil via C(sp3)-H bond activation mediated by Selectfluor
- PMID: 35520934
- PMCID: PMC9062302
- DOI: 10.1039/c9ra01481k
An efficient 3-acylquinoline synthesis from acetophenones and anthranil via C(sp3)-H bond activation mediated by Selectfluor
Abstract
An efficient method for the synthesis of 3-functionalized quinolines from commercially available acetophenones and anthranil has been described. Selectfluor propels the C(sp3)-H bond activation of the acetophenones and aza-Michael addition of anthranil resulting in annulated 3-acylquinolines in moderate to high yields. DMSO acts not only as a solvent but also as a one carbon donor in the reaction.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Transition-Metal-Free Quinoline Synthesis from Acetophenones and Anthranils via Sequential One-Carbon Homologation/Conjugate Addition/Annulation Cascade.Org Lett. 2017 Sep 15;19(18):4948-4951. doi: 10.1021/acs.orglett.7b02429. Epub 2017 Sep 7. Org Lett. 2017. PMID: 28880095
-
Selectfluor-Mediated Synthesis of β-Acyl Allyl Sulfones/β-Acyl Allyl Benzotriazoles from Ketones/Acetylenes, Aryl Sulfinates/Benzotriazole, and DMSO as a Dual-Carbon Synthon.J Org Chem. 2022 Mar 4;87(5):2435-2445. doi: 10.1021/acs.joc.1c02348. Epub 2022 Jan 13. J Org Chem. 2022. PMID: 35025500
-
C-H Bond Functionalization of N-Heteroarenes Mediated by Selectfluor.Top Curr Chem (Cham). 2023 Sep 22;381(5):29. doi: 10.1007/s41061-023-00437-6. Top Curr Chem (Cham). 2023. PMID: 37736818 Review.
-
Copper(II)-Catalyzed Selective CAr-H Bond Formylation: Synthesis of Dialdehyde Aniline.Front Chem. 2022 May 24;10:891858. doi: 10.3389/fchem.2022.891858. eCollection 2022. Front Chem. 2022. PMID: 35685349 Free PMC article.
-
Organocatalytic Synthesis of Highly Functionalized Heterocycles by Enantioselective aza-Morita-Baylis-Hillman-Type Domino Reactions.Chem Pharm Bull (Tokyo). 2020;68(4):299-315. doi: 10.1248/cpb.c19-00900. Chem Pharm Bull (Tokyo). 2020. PMID: 32238648 Review.
Cited by
-
Recent Advances in the Use of Dimethyl Sulfoxide as a Synthon in Organic Chemistry.Top Curr Chem (Cham). 2022 Oct 28;380(6):55. doi: 10.1007/s41061-022-00411-8. Top Curr Chem (Cham). 2022. PMID: 36306032 Review.
References
-
- Jampilek J. Curr. Org. Chem. 2017;21:1824–1846. doi: 10.2174/1385272821666161214121512. - DOI
-
- Arnison P. G. Bibb M. J. Bierbaum G. Bowers A. A. Bugni T. S. Bulaj G. Camarero J. A. Campopiano D. J. Challis G. L. Clardy J. Cotter P. D. Craik D. J. Dawson M. Dittmann E. Donadio S. Dorrestein P. C. Entian K.-D. Fischbach M. A. Garavelli J. S. Goeransson U. Gruber C. W. Haft D. H. Hemscheidt T. K. Hertweck C. Hill C. Horswill A. R. Jaspars M. Kelly W. L. Klinman J. P. Kuipers O. P. Link A. J. Liu W. Marahiel M. A. Mitchell D. A. Moll G. N. Moore B. S. Mueller R. Nair S. K. Nes I. F. Norris G. E. Olivera B. M. Onaka H. Patchett M. L. Piel J. Reaney M. J. T. Rebuffat S. Ross R. P. Sahl H.-G. Schmidt E. W. Selsted M. E. Severinov K. Shen B. Sivonen K. Smith L. Stein T. Suessmuth R. D. Tagg J. R. Tang G.-L. Truman A. W. Vederas J. C. Walsh C. T. Walton J. D. Wenzel S. C. Willey J. M. van der Donk W. A. Nat. Prod. Rep. 2013;30:108–160. doi: 10.1039/C2NP20085F. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources