Facile synthesis of g-C3N4 with various morphologies for application in electrochemical detection
- PMID: 35521191
- PMCID: PMC9061280
- DOI: 10.1039/c8ra10166c
Facile synthesis of g-C3N4 with various morphologies for application in electrochemical detection
Abstract
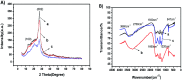
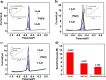
In the present study, g-C3N4 with various morphologies was successfully synthesized via a variety of facile in situ methods. The as-prepared products were characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), Raman spectroscopy and X-ray diffraction (XRD). The results obtained using square wave anodic stripping voltammetry (SWASV) showed that when g-C3N4 was applied as an electrochemical sensor, it exhibited excellent sensitivity and selectivity for the detection of heavy metal ions including Pb(ii), Cu(ii) and Hg(ii). Compared to nanoporous graphitic carbon nitride (npg-C3N4) and g-C3N4 nanosheet-modified glass carbon electrode (GCE), g-C3N4 successfully realized the individual and simultaneous detection of four target heavy ions for the first time. In particular, g-C3N4 displayed significant electrocatalytic activity towards Hg(ii) with a good sensitivity of 18.180 μA μM-1 and 35.923 μA μM-1 under the individual and simultaneous determination conditions, respectively. The sensitivity for simultaneous determination was almost 2 times that of the individual determination. Moreover, the fabricated electrochemical sensor showed good anti-interference, stability and repeatability; this indicated significant potential of the proposed materials for application in high-performance electrochemical sensors for the individual and simultaneous detection of heavy metal ions.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures









References
-
- Gumpua M. B. Sethuraman S. Krishnan U. M. Rayappan J. B. B. Sens. Actuators, B. 2015;213:515–533. doi: 10.1016/j.snb.2015.02.122. - DOI
-
- Aragay G. Merkoc A. Electrochim. Acta. 2012:8449–8461.
LinkOut - more resources
Full Text Sources

