Encapsulated actomyosin patterns drive cell-like membrane shape changes
- PMID: 35521522
- PMCID: PMC9061794
- DOI: 10.1016/j.isci.2022.104236
Encapsulated actomyosin patterns drive cell-like membrane shape changes
Abstract
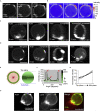
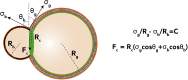
Cell shape changes from locomotion to cytokinesis are, to a large extent, driven by myosin-driven remodeling of cortical actin patterns. Passive crosslinkers such as α-actinin and fascin as well as actin nucleator Arp2/3 complex largely determine actin network architecture and, consequently, membrane shape changes. Here we reconstitute actomyosin networks inside cell-sized lipid bilayer vesicles and show that depending on vesicle size and concentrations of α-actinin and fascin actomyosin networks assemble into ring and aster-like patterns. Anchoring actin to the membrane does not change actin network architecture yet exerts forces and deforms the membrane when assembled in the form of a contractile ring. In the presence of α-actinin and fascin, an Arp2/3 complex-mediated actomyosin cortex is shown to assemble a ring-like pattern at the equatorial cortex followed by myosin-driven clustering and consequently blebbing. An active gel theory unifies a model for the observed membrane shape changes induced by the contractile cortex.
Keywords: Biological sciences; Cell biology; Mechanobiology.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Bashirzadeh Y., Liu A.P. Encapsulation of the cytoskeleton: towards mimicking the mechanics of a cell. Soft Matter. 2019;15:8425–8436. - PubMed
-
- Bashirzadeh Y., Qian S., Maruthamuthu V. Non-intrusive measurement of wall shear stress in flow channels. Sensors Actuators A: Phys. 2018;271:118–123. doi: 10.1016/J.SNA.2018.01.012. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

