Organic acid cross-linked 3D printed cellulose nanocomposite bioscaffolds with controlled porosity, mechanical strength, and biocompatibility
- PMID: 35521531
- PMCID: PMC9062678
- DOI: 10.1016/j.isci.2022.104263
Organic acid cross-linked 3D printed cellulose nanocomposite bioscaffolds with controlled porosity, mechanical strength, and biocompatibility
Abstract
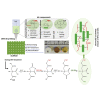
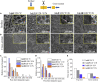
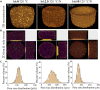
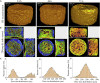
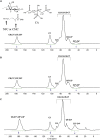
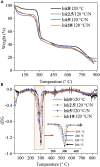
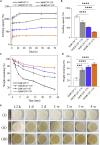
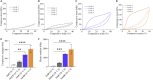
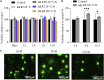
Herein, we fabricated chemically cross-linked polysaccharide-based three-dimensional (3D) porous scaffolds using an ink composed of nanofibrillated cellulose, carboxymethyl cellulose, and citric acid (CA), featuring strong shear thinning behavior and adequate printability. Scaffolds were produced by combining direct-ink-writing 3D printing, freeze-drying, and dehydrothermal heat-assisted cross-linking techniques. The last step induces a reaction of CA. Degree of cross-linking was controlled by varying the CA concentration (2.5-10.0 wt.%) to tune the structure, swelling, degradation, and surface properties (pores: 100-450 μm, porosity: 86%) of the scaffolds in the dry and hydrated states. Compressive strength, elastic modulus, and shape recovery of the cross-linked scaffolds increased significantly with increasing cross-linker concentration. Cross-linked scaffolds promoted clustered cell adhesion and showed no cytotoxic effects as determined by the viability assay and live/dead staining with human osteoblast cells. The proposed method can be extended to all polysaccharide-based materials to develop cell-friendly scaffolds suitable for tissue engineering applications.
Keywords: Biomaterials; Materials science; Tissue Engineering.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Abbasi N., Hamlet S., Love R.M., Nguyen N.-T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices. 2020;5:1–9. doi: 10.1016/j.jsamd.2020.01.007. - DOI
-
- Bahrami N., Farzin A., Bayat F., Goodarzi A., Salehi M., Karimi R., Mohamadnia A., Parhiz A., Ai J. Optimization of 3D alginate scaffold properties with interconnected porosity using freeze-drying method for cartilage tissue engineering application. Arch. Neurosci. 2019;6 doi: 10.5812/ans.85122. - DOI
-
- Berillo D., Volkova N. Preparation and physicochemical characteristics of cryogel based on gelatin and oxidised dextran. J. Mater. Sci. 2014;49:4855–4868. doi: 10.1007/s10853-014-8186-3. - DOI
-
- Bose S., Vahabzadeh S., Bandyopadhyay A. Bone tissue engineering using 3D printing. Mater. Today. 2013;16:496–504. doi: 10.1016/j.mattod.2013.11.017. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

