L3MBTL2-mediated CGA transcriptional suppression promotes pancreatic cancer progression through modulating autophagy
- PMID: 35521536
- PMCID: PMC9061862
- DOI: 10.1016/j.isci.2022.104249
L3MBTL2-mediated CGA transcriptional suppression promotes pancreatic cancer progression through modulating autophagy
Abstract
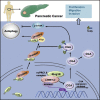
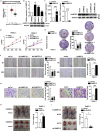
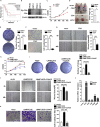
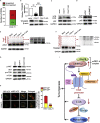
L3MBTL2 is a crucial component of ncPRC1.6 and has been implicated in transcriptional repression and chromatin compaction. However, the repression mechanism of L3MBTL2 and its biological functions are largely undefined. Here, we found that L3MBTL2 plays a distinct oncogenic role in tumor development. We demonstrated that L3MBTL2 repressed downstream CGA through an H2AK119ub1-dependent mechanism. Importantly, the binding of the MGA/MAX heterodimer to the E-box on the CGA promoter enhanced the specific selective repression of CGA by L3MBTL2. CGA encodes the alpha subunit of glycoprotein hormones; however, we showed that CGA plays an individual tumor suppressor role in PDAC. Moreover, CGA-transcript1 (T1) was identified as the major transcript, and the tumor suppression function of CGA-T1 depends on its own glycosylation. Furthermore, glycosylated CGA-T1 inhibited PDAC, partly by repression of autophagy through multiple pathways, including PI3K/Akt/mTOR and TP53INP2 pathways. These findings reveal the important roles of L3MBTL2 and CGA in tumor development.
Keywords: Biological sciences; Cancer; Cell biology.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests
Figures




References
-
- Bieche I., Parfait B., Le Doussal V., Olivi M., Rio M.C., Lidereau R., Vidaud M. Identification of CGA as a novel estrogen receptor-responsive gene in breast cancer: an outstanding candidate marker to predict the response to endocrine therapy. Cancer Res. 2001;61:1652–1658. - PubMed
-
- Blackledge N.P., Farcas A.M., Kondo T., King H.W., Mcgouran J.F., Hanssen L.L.P., Ito S., Cooper S., Kondo K., Koseki Y., et al. Variant PRC1 complex-dependent H2A ubiquitylation drives PRC2 recruitment and polycomb domain formation. Cell. 2014;157:1445–1459. doi: 10.1016/j.cell.2014.05.004. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

