Mechanistic insights into multiple-step transport of mitochondrial ADP/ATP carrier
- PMID: 35521544
- PMCID: PMC9046947
- DOI: 10.1016/j.csbj.2022.03.032
Mechanistic insights into multiple-step transport of mitochondrial ADP/ATP carrier
Abstract
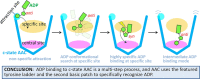
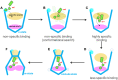
The ADP/ATP carrier (AAC) is crucial for mitochondrial functions by importing ADP and exporting ATP across the inner mitochondrial membrane. However, the mechanism of highly specific ADP recognition and transport by AAC remains largely elusive. In this work, spontaneous ADP binding process to the ground c-state AAC was investigated through rigorous molecular dynamics simulations of over 31 microseconds in total. With improved simulation strategy, we have successfully identified a highly specific ADP binding site in the upper region of the cavity, and this site exhibits selectivity for ADP over ATP based on free-energy calculations. Sequence analyses on adenine nucleotide transporters also suggest that this subgroup uses the upper region of the cavity, rather than the previously proposed central binding site located at the bottom of the cavity to discriminate their substrates. Identification of the new site unveils the unusually high substrate specificity of AAC and explains the dependence of transport on the flexibility between anti and syn glycosidic conformers of ADP. Moreover, this new site together with the central site supports early biochemical findings. In light of these early findings, our simulations described a multi-step model in which the carrier uses different sites for substrate attraction, recognition and conformational transition. These results provide new insights into the transport mechanism of AAC and other adenine nucleotide transporters.
Keywords: AAC, ADP/ADP carrier; ATP translocases; CATR, carboxyatractyloside; CoA, coenzyme A; GDC, Graves disease carrier protein, or SLC25A16; MCF, mitochondrial carrier family; MD simulation, molecular dynamics simulation PCA, Principal component analysis; Mitochondrial ADP; OXPHOS, oxidative phosphorylation; SCaMCs, short Ca2+-binding mitochondrial carrier, or Mg-ATP/Pi carrier; Solute carrier family 25, molecular dynamics simulation; Substrate recognition; Transporter; c-state, cytosol-open state; m-state, matrix-open state.
© 2022 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
